| CPC A61K 31/4184 (2013.01) [A61K 31/4192 (2013.01); A61K 31/423 (2013.01); A61K 31/428 (2013.01); A61K 31/4745 (2013.01); A61P 35/00 (2018.01); A61P 35/02 (2018.01); C07D 263/60 (2013.01); C07D 401/04 (2013.01); C07D 405/04 (2013.01); C07D 413/04 (2013.01); C07D 417/04 (2013.01)] | 11 Claims |
|
1. A method for treating solid cancer or blood cancer, comprising administering to a patient in need thereof a therapeutically effective amount of a 1,2-naphotoquinone derivative compound represented by Formula 1 below or a pharmaceutically acceptable salt thereof:
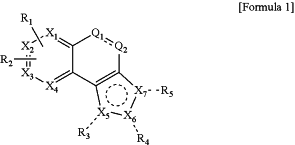 wherein,
R1 and R2 are each independently hydrogen, halogen, hydroxy, C1-C6 alkoxy, C1-C6 alkyl, —NO2, —NR′1R′2, —NR′1(CO(O)R′2), —NR′1(C(O)NR′1R′2), —CN, wherein R′1 and R′2 are each independently hydrogen, or C1-C6 alkyl;
R3 is hydrogen, halogen, hydroxy, C1-C6 alkyl, C1-C6 alkoxy, C3-C8 cycloalkyl, (CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—OR′3, or —CO(O)R′3, wherein R′3 is hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryloxy, —(CR′5R′6)m—C1-C10 heteroaryl, or —CO(O)R″3, wherein R′5 and R′6 are each independently hydrogen or C1-C3 alkyl, and R″3 is C1-C6 alkyl;
R4 is hydrogen, halogen, hydroxy, C1-C6 alkyl, C2-C10 alkene, C1-C6 alkoxy, C3-C8 cycloalkyl, C2-C8 heterocycloalkyl, C4-C10 aryl, C4-C10 aryloxy, C1-C8 heteroaryl, —(CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryloxy, —(CR′5R′6)m—C1-C8 heteroaryl, —(CR′5R′6)m—NR′3R′4, —(CR′5R′6)m—C3-C8 heterocycloalkyl, —(CR′5R′6)m—OR′3, —(CR′5R′6)m(O)COR′3, —CO(O)R′3, —CONR′3R′4, —NR′3R′4, or —NR′3(C(O)R′4), wherein R′3 and R′4 are each independently hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryloxy, —(CR′5R′6)m—C1-C10 heteroaryl, or —CO(O)R″3, or R′3 and R′4 may form a cyclic structure of C2-C10 heterocycloalkyl or a cyclic structure of C1-C10 heteroaryl by mutual coupling, and R′5 and R′6 are each independently hydrogen or C1-C3 alkyl, wherein R″3 is C1-C6 alkyl;
R5 is hydrogen, halogen, hydroxy, C1-C6 alkyl, C2-C10 alkene, C1-C6 alkoxy, C3-C8 cycloalkyl, C2-C8 heterocycloalkyl, C4-C10 aryl, C4-C10 aryloxy, C1-C8 heteroaryl, —(CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryloxy, —(CR′5R′6)m—C1-C8 heteroaryl, —(CR′5R′6)m—NR′3R′4, —(CR′5R′6)m—C3-C8 heterocycloalkyl, —(CR′5R′6)m—OR′3, —(CR′5R′6)m(0)COR′3, —CO(O)R′3, —CONR′3R′4, —NR′3R′4, or —NR′3(C(O)R′4), wherein R′3 and R′4 are each independently hydrogen, C1-C6 alkyl, C3-C8 cycloalkyl, C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryl, —(CR′5R′6)m—C4-C10 aryloxy, —(CR′5R′6)m—C1-C10 heteroaryl, or —CO(O)R″3, or R′3 and R′4 may form a cyclic structure of C2-C10 heterocycloalkyl or a cyclic structure of C1-C10 heteroaryl by mutual coupling, and R's and R′6 are each independently hydrogen or C1-C3 alkyl, wherein R″3 is C1-C6 alkyl,
Q1 and Q2 are each CO;
m and m′ are each independently an integer of 1 to 4;
a heteroatom is at least one selected from N, O, and S;
X1 to X4 are each independently CH or N(R″6), wherein R″6 is hydrogen or C1-C3 alkyl;
X5, X6, and X7 are N; and
the sign ---- represents a single bond or represents that a bond may not be formed, and the sign
 in the Formula 1 represents that a cyclic structure containing the sign may be or may not be an aromatic structure.
|