| CPC A61K 31/137 (2013.01) [A61K 9/2031 (2013.01); A61K 9/2866 (2013.01); A61K 9/5026 (2013.01); A61K 9/5047 (2013.01); A61K 47/542 (2017.08); A61K 47/64 (2017.08)] | 32 Claims |
|
1. A process for preparing a drug substance comprising the steps of:
(i) providing a substituted amphetamine or a pharmaceutically acceptable salt, solvate, or mixture of two or more thereof, as an active pharmaceutical ingredient, wherein the substituted amphetamine comprises not more than 0.1% by weight of amphetamine-process related impurity,
wherein the substituted amphetamine is produced by a process that comprises the steps of performing a stereospecific cuprate addition reaction upon an aziridine phosphoramidate compound to obtain a aryl or aryl-alkyl phosphoramidate amphetamine precursor, and deprotecting the aryl or aryl-alkyl phosphoramidate amphetamine precursor under acidic conditions effective to produce a substituted amphetamine,
wherein the substituted amphetamine is selected from the group consisting of: dex-amphetamine, dex-N-methylamphetamine, and dex-N-ethylamphetamine, and a racemic mixture of amphetamine isomers, wherein the dex-amphetamine, dex-N-methylamphetamine, dex-N-ethylamphetamine, and racemic mixture of amphetamine isomers is made according to the process comprising the steps 1a and 2a:
(1a) providing a compound of Formula 5:
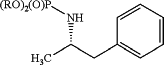 wherein R is alkyl or aryl; and
(2a) deprotecting the compound of Formula 5 under acidic conditions effective to produce (2S)-1-phenylpropan-2-amine of Formula I:
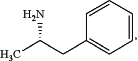 and wherein the racemic mixture of amphetamine isomers is made according to the process comprising the steps 1b and 2b:
(1b) providing a compound of Formula 6:
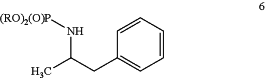 wherein R is alkyl or aryl; and
(2b) deprotecting the compound of Formula 6 under acidic conditions effective to produce a racemic mixture of amphetamine isomers of Formula 7:
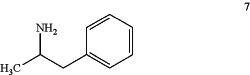 further comprising the step of formulating the drug substance with one or more excipients to produce a pharmaceutical composition
wherein the regioisomeric purity of Formula 5 is >99% and the regioisomer is <0.1%.
|