| CPC A61B 18/1492 (2013.01) [A61B 18/1206 (2013.01); A61N 1/0551 (2013.01); A61N 1/36017 (2013.01); A61N 1/36031 (2017.08); A61N 1/36103 (2013.01); A61N 1/36135 (2013.01); A61B 18/082 (2013.01); A61B 18/16 (2013.01); A61B 2018/0016 (2013.01); A61B 2018/00345 (2013.01); A61B 2018/00434 (2013.01); A61B 2018/00505 (2013.01); A61B 2018/00511 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00714 (2013.01); A61B 2018/00726 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00863 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/00994 (2013.01); A61B 2018/124 (2013.01); A61B 2018/126 (2013.01); A61B 2018/1253 (2013.01); A61B 2018/1407 (2013.01); A61B 2018/1435 (2013.01); A61B 2018/1467 (2013.01); A61B 2218/002 (2013.01); A61N 1/0558 (2013.01)] | 22 Claims |
|
1. A system comprising:
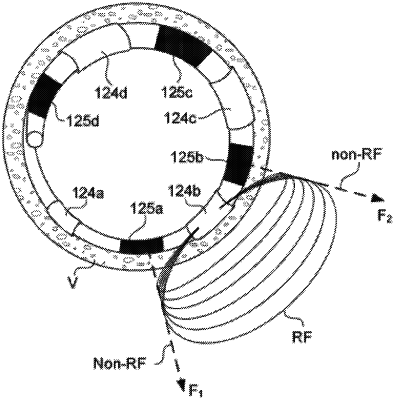
a catheter comprising an elongated member configured to be navigated through a vasculature of a patient, wherein the elongated member comprises an electrode array, the electrode array including:
a plurality of primary electrodes spaced apart along the elongated member, and
a plurality of secondary electrodes spaced apart along the elongated member; and
a console configured to be electrically coupled to the plurality of primary electrodes and the plurality of secondary electrodes, wherein the console is configured to:
generate and deliver a radiofrequency (RF) electric field via at least one primary electrode of the plurality of primary electrodes, wherein the RF electric field is configured to ablate nerves at or adjacent a target tissue site, and
generate and deliver a non-ablative electric field via at least one secondary electrode of the plurality of secondary electrodes substantially simultaneously with the RF electric field to at least partially reduce transmission of the RF electric field, wherein the non-ablative electric field is configured to avoid ablating the nerves at or adjacent the target tissue site.
|