| CPC C10G 45/08 (2013.01) [B01J 21/04 (2013.01); B01J 23/75 (2013.01); B01J 23/88 (2013.01); B01J 35/006 (2013.01); B01J 35/023 (2013.01); B01J 35/1019 (2013.01); B01J 35/1038 (2013.01); B01J 35/1061 (2013.01); B01J 37/0207 (2013.01); C10G 45/14 (2013.01); C10G 2300/1055 (2013.01); C10G 2300/202 (2013.01); C10G 2300/70 (2013.01); C10G 2400/26 (2013.01)] | 10 Claims |
|
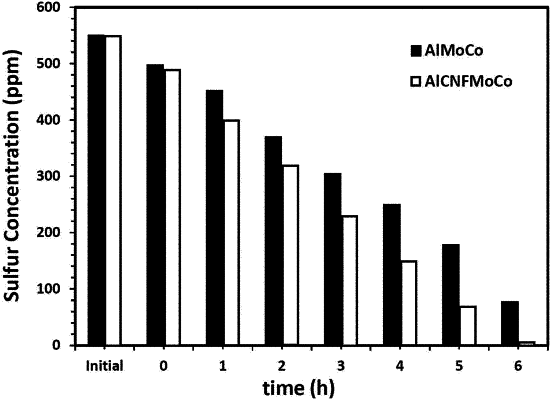
1. A method for removing sulfur from a liquid fuel comprising one or more organosulfur compounds, comprising:
contacting the liquid fuel with a hydrodesulfurization catalyst in a reactor in the presence of hydrogen gas, thereby forming a fuel mixture comprising less sulfur than the liquid fuel,
wherein the contacting is carried out at a temperature in a range of from 250 to 350° C. for up to 6 hours and a hydrogen gas partial pressure in a range of from 50 to 60 bar-a,
wherein the hydrodesulfurization catalyst comprises:
catalytic material comprising molybdenum and cobalt; and
a catalyst support comprising alumina;
wherein the catalyst support further comprises carbon nanofibers dispersed on a surface of the alumina; and
wherein the hydrodesulfurization catalyst further comprises a dopant comprising boron, and
wherein the hydrodesulfurization catalytic material is homogenously dispersed on the catalyst support.
|