| CPC C07D 403/12 (2013.01) [C07B 2200/13 (2013.01)] | 32 Claims |
|
1. A crystalline salt of Compound I:
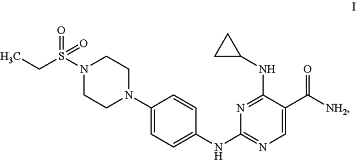 (a) wherein the salt is esylate, characterized by an X-ray powder diffractogram comprising peaks at 7.5, 18.1, and 14.9 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I esylate Type A);
(b) wherein the salt is mesylate, characterized by an X-ray powder diffractogram comprising peaks at 16.0, 16.4, and 16.7 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I mesylate Type A);
(c) wherein the salt is isethionate, characterized by an X-ray powder diffractogram comprising peaks at 15.8, 17.9, and 20.1 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I isethionate Type A);
(d) wherein the salt is naphthalenesulfonate, characterized by an X-ray powder diffractogram comprising peaks at 14.4, 20.2, and 7.5 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I naphthalenesulfonate Type A);
(e) wherein the salt is naphthalenesulfonate, characterized by an X-ray powder diffractogram comprising peaks at 9.0, 15.6, and 19.7 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I naphthalenesulfonate Type B);
(f) wherein the salt is naphthalenesulfonate, characterized by an X-ray powder diffractogram comprising peaks at 6.9, 13.6, and 13.3 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I naphthalenesulfonate Type C)
(g) wherein the salt is besylate, characterized by an X-ray powder diffractogram comprising peaks at 7.4, 14.9, and 15.3 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I besylate Type A);
(h) wherein the salt is besylate, characterized by an X-ray powder diffractogram comprising peaks at 6.0, 11.9, and 6.9 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I besylate Type B)
(i) wherein the salt is edisylate, characterized by an X-ray powder diffractogram comprising peaks at 14.7, 24.5, and 19.1 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I edisylate Type D);
(j) wherein the salt is camphorsulfonate, characterized by an X-ray powder diffractogram comprising peaks at 5.9, 14.3, and 17.8 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I camphorsulfonate Type A);
(k) wherein the salt is camphorsulfonate, characterized by an X-ray powder diffractogram comprising peaks at 4.7, 14.0, and 17.2 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I camphorsulfonate Type B);
(l) wherein the salt is camphorsulfonate, characterized by an X-ray powder diffractogram comprising peaks at 13.5, 16.8, and 9.5 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I camphorsulfonate Type C);
(m) wherein the salt is chlorobenzenesulfate, characterized by an X-ray powder diffractogram comprising peaks at 8.8, 18.5, and 9.5 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I chlorobenzenesulfate Type B);
(n) wherein the salt is tosylate, characterized by an X-ray powder diffractogram comprising peaks at 7.5, 15.0, and 8.9 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I tosylate Type C); or
(o) wherein the salt is tosylate, characterized by an X-ray powder diffractogram comprising peaks at 9.2, 19.2, and 6.3 °2θ, each ±0.2 °2θ, as determined on a diffractometer using Cu-Kα radiation (Compound I tosylate Type D).
|