| CPC B01J 23/464 (2013.01) [B01J 35/0033 (2013.01); B01J 35/023 (2013.01); B01J 35/08 (2013.01); B01J 35/1019 (2013.01); B01J 37/0209 (2013.01); B01J 37/08 (2013.01); C07C 209/32 (2013.01); B01J 23/745 (2013.01); C07C 211/45 (2013.01); C07C 211/50 (2013.01)] | 15 Claims |
|
1. A process of reducing an aromatic nitro compound to an aromatic amine compound, the method comprising:
mixing the aromatic nitro compound with a hydrogen transfer reagent in the presence of a supported catalyst and a solvent to form a reaction mixture; and
heating the reaction mixture thereby forming the aromatic amine compound;
wherein the supported catalyst comprises:
a support material comprising Fe3O4; and
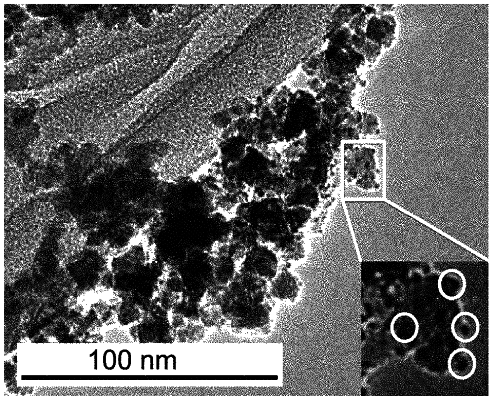
a catalytic material comprising rhodium disposed on the support material;
wherein the catalytic material is in the form of subnanoparticles having an average particle size of 0.2-0.99 nm;
the rhodium is present in an amount of 1-20 wt % relative to a total weight of the supported catalyst; and
the support material is devoid of Al2O3.
|