|
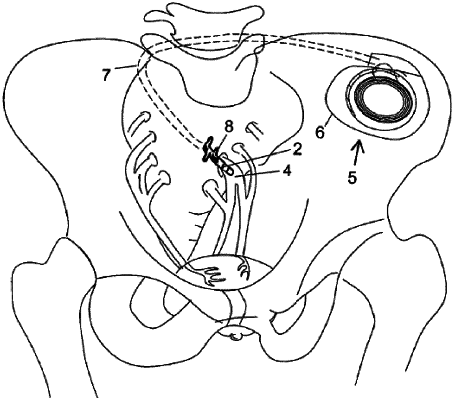
1. A method comprising transcutaneously measuring motility patterns, in the colon of a recipient of an implantable neuromodulator, responsive to an electrical stimulus delivered by the implantable neuromodulator, and programming the implantable neuromodulator responsive to the measured motility patterns, wherein the method comprises adjusting at least one parameter of the implantable neuromodulator that defines the electrical stimulus that the implantable neuromodulator is configured to deliver to the recipient, wherein the method comprises transcutaneously measuring the response of smooth muscle in the colon to electrical stimulation of the spinal cord or the sacral nerve.
|