| CPC A61K 48/0033 (2013.01) [A61K 9/127 (2013.01); A61K 47/18 (2013.01); C07C 219/06 (2013.01); C07C 229/12 (2013.01); C07C 229/16 (2013.01); C07C 233/18 (2013.01); C07C 255/24 (2013.01); C12N 9/0069 (2013.01); C12Y 113/12007 (2013.01)] | 35 Claims |
|
1. A lipid nanoparticle comprising:
i) from 47 to 48 mol percent of a cationic lipid;
ii) a neutral lipid;
iii) a steroid;
iv) a polymer conjugated lipid; and
v) a nucleic acid, or a pharmaceutically acceptable salt thereof, encapsulated within or associated with the lipid nanoparticle,
wherein the cationic lipid has a structure of Formula II:
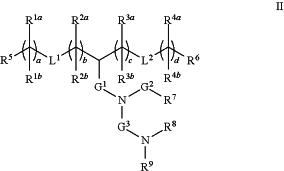 or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, wherein:
one of L1 or L2 is —O(C═O)—, —(C═O)O—, —C(═O)—, —O—, —S(O)x—, —S—S—, —C(═O)S—, SC(═O)—, —NRaC(═O)—, —C(═O)NRa—, NRaC(═O)NRa—, —OC(═O)NRa— or —NRaC(═O)O—, and the other of L1 or L2 is —O(C═O)—, —(C═O)O—, —C(═O)—, —O—, —S(O)—, —S—S—, —C(═O)S—, SC(═O)—, —NRaC(═O)—, —C(═O)NRa—, NRaC(═O)NRa—, —OC(═O)NRa— or —NRaC(═O)O— or a direct bond;
G1 is C1-C2 alkylene, —(C═O)—, —O(C═O)—, —SC(═O)—, —NRaC(═O)— or a direct bond;
G2 is —C(═O)—, —(C═O)O—, —C(═O)S—, —C(═O)NRa— or a direct bond;
G3 is C1-C6 alkylene;
Ra is H or C1-C12 alkyl;
R1a and R1b are, at each occurrence, independently either: (a) H or C1-C12 alkyl; or (b) R1a is H or C1-C12 alkyl, and R1b together with the carbon atom to which it is bound is taken together with an adjacent R1b and the carbon atom to which it is bound to form a carbon-carbon double bond;
R2a and R2b are, at each occurrence, independently either: (a) H or C1-C12 alkyl; or (b) R2a is H or C1-C12 alkyl, and R2b together with the carbon atom to which it is bound is taken together with an adjacent R2b and the carbon atom to which it is bound to form a carbon-carbon double bond;
R3a and R3b are, at each occurrence, independently either (a): H or C1-C12 alkyl; or (b) R3a is H or C1-C12 alkyl, and R3b together with the carbon atom to which it is bound is taken together with an adjacent R3b and the carbon atom to which it is bound to form a carbon-carbon double bond;
R4a and R4b are, at each occurrence, independently either: (a) H or C1-C12 alkyl; or (b) R4a is H or C1-C12 alkyl, and R4b together with the carbon atom to which it is bound is taken together with an adjacent R4b and the carbon atom to which it is bound to form a carbon-carbon double bond;
R5 and R6 are each independently H or methyl;
R7 is C4-C20 alkyl;
R8 and R9 are each independently C1-C12 alkyl; or R8 and R9, together with the nitrogen atom to which they are attached, form a 5, 6 or 7-membered heterocyclic ring;
a, b, c and d are each independently an integer from 1 to 24; and
x is 0, 1 or 2.
|