| CPC A61K 47/44 (2013.01) [A61K 9/1272 (2013.01); A61K 31/7088 (2013.01); A61K 31/713 (2013.01); A61K 39/00 (2013.01); A61K 39/39 (2013.01); A61K 47/10 (2013.01); A61K 47/18 (2013.01); A61K 47/20 (2013.01); A61K 47/28 (2013.01); C07C 229/08 (2013.01); C07C 229/30 (2013.01); C07C 237/16 (2013.01); C07C 251/38 (2013.01); C07C 251/78 (2013.01); C07C 271/12 (2013.01); C07C 271/20 (2013.01); C07C 323/25 (2013.01); C07D 203/10 (2013.01); C07D 317/28 (2013.01); C07D 317/44 (2013.01); C07D 317/46 (2013.01); C07D 317/72 (2013.01); C07D 319/06 (2013.01); C07D 405/12 (2013.01); C07D 491/056 (2013.01); C07D 491/113 (2013.01); C12N 15/111 (2013.01); C12N 15/113 (2013.01); A61K 2039/55555 (2013.01); A61K 2039/55561 (2013.01); C12N 2310/14 (2013.01); C12N 2310/3515 (2013.01); C12N 2320/32 (2013.01); Y02A 50/30 (2018.01)] | 16 Claims |
|
1. A lipid having the structure
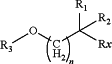 E is C(O)O or OC(O);
R1 and R2 and Rx are each independently for each occurrence H, optionally substituted C1-C10 alkyl, or optionally substituted C10-C30 acyl, provided that at least one of R1, R2 and Rx is not H;
R3 is optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, alkylheterocycle, alkylphosphate, alkylphosphorothioate, alkylphosphorodithioate, alkylphosphonate, alkylamine, hydroxyalkyl, ω-aminoalkyl, ω-(substituted)aminoalkyl, ω-phosphoalkyl, ω-thiophosphoalkyl heteroaryl, or heterocycle; and
n is 0, 1, 2, or 3.
|