| CPC A61K 9/0053 (2013.01) [A61K 31/4704 (2013.01); A61K 31/496 (2013.01); A61K 31/5377 (2013.01)] | 11 Claims |
|
1. An oral pharmaceutical composition, comprising a pharmaceutically acceptable salt of a prodrug of rebamipide, as an active ingredient,
wherein the prodrug of rebamipide is 2-morpholinoethyl 2-(4-chlorobenzamido)-3-(2-oxo-1,2-dihydroquinolin-4-yl)propanoate represented by Chemical Formulae II, and
wherein the pharmaceutically acceptable salt is a sulfate of a prodrug of rebamipide, a malonate of a prodrug of rebamipide, or an oxalate of a prodrug of rebamipide:
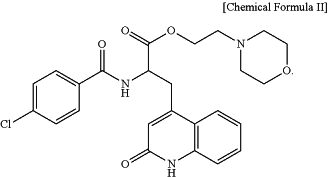 |