| CPC A61F 5/0036 (2013.01) [A61L 15/60 (2013.01); A61L 31/148 (2013.01)] | 20 Claims |
|
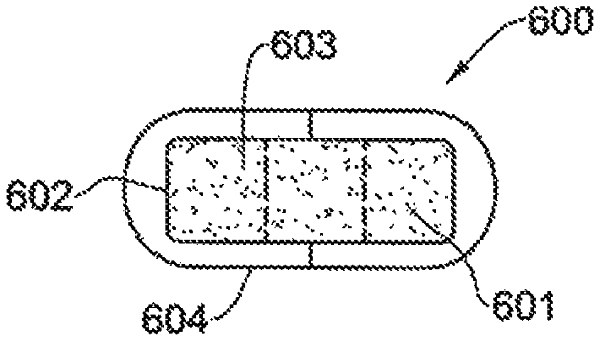
1. A folded expandable gastro-retentive device having an initial folded form, said form being transformable, in sequence, upon delivery to a subject gastrointestinal system, into (a) an expanded 3-dimensional form, (b) a declined form, and (c) a disintegrated form,
said device comprising at least one compartment having an external enteric-biodegradable film that allows stomach liquid permeation therethrough, said at least one compartment enclosing a film that comprises at least one gel-forming compound;
wherein said at least one gel-forming compound is configured to undergo swelling into a gel upon contact with liquid in the stomach to transform said device into the expanded, 3-dimensional form (a);
wherein said at least one gel-forming compound is configured to, upon continued stomach residence, undergo at least partial degradation thereby causing the device in the expanded form (a) to adopt the declined form (b) and evacuate through the pylorus into the intestine; and
wherein the external enteric biodegradable film is configured to enterically degrade in the intestine to transform the device to the disintegrated form (c).
|