| CPC C25F 7/00 (2013.01) [C25B 3/26 (2021.01); C25B 11/061 (2021.01); C25D 11/34 (2013.01); C25D 21/12 (2013.01); C25F 3/26 (2013.01)] | 8 Claims |
|
1. An electrochemical method comprising:
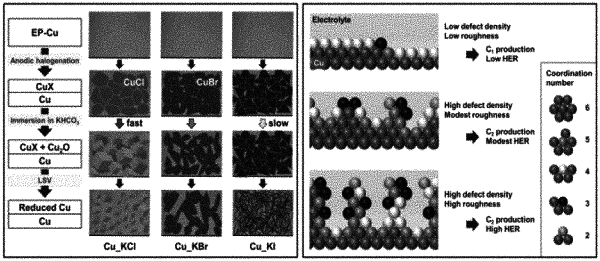
performing anodic halogenation of Cu foils; wherein the anodic halogenation comprises 60-100 s at 1.1 V (vs. Ag/AgCl) for KCI, 60-90 s at 0.18 V (vs. Ag/AgCl) for KBr, or 1-10 s at −0.2 V (vs. Ag/AgCl) for KI;
performing subsequent oxide-formation in a KHCO3 electrolyte; and
performing an electroreduction in neutral KHCO3 to generate a copper catalyst comprising cubic structures of Cu and a surface roughness of less than 30; the catalyst capable to provide a faradaic efficiency ≥50% for C2H4 generation.
|