| CPC C07D 487/04 (2013.01) | 16 Claims |
|
1. A solid form of Compound (I):
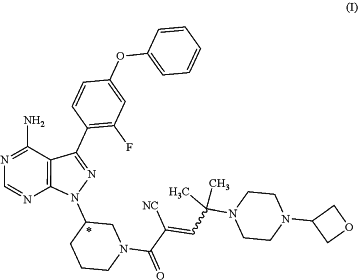 wherein the solid form is characterized by a mean bulk density greater than 0.3 g/cc.
|
|
9. A pharmaceutical composition comprising at least one pharmaceutically acceptable excipient and the solid form according to claim 1.
|