| CPC C07D 207/12 (2013.01) [A61K 31/40 (2013.01); A61K 31/445 (2013.01); A61K 31/4427 (2013.01); A61K 31/454 (2013.01); A61K 31/4545 (2013.01); A61K 45/06 (2013.01); A61P 37/02 (2018.01); C07D 205/04 (2013.01); C07D 205/12 (2013.01); C07D 207/267 (2013.01); C07D 211/38 (2013.01); C07D 211/40 (2013.01); C07D 211/44 (2013.01); C07D 211/46 (2013.01); C07D 211/76 (2013.01); C07D 213/85 (2013.01); C07D 295/088 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 451/06 (2013.01); C07D 471/10 (2013.01); C07D 487/10 (2013.01); C07F 5/025 (2013.01)] | 25 Claims |
|
1. A compound of Formula (I)
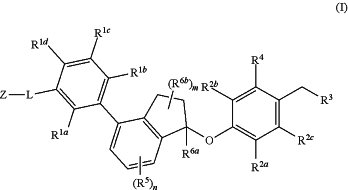 or a pharmaceutically acceptable salt thereof, wherein:
each of R1a, R1b, R1c, and R1d is independently selected from the group consisting of H, halogen, CF3, CN, C1-4 alkyl, and —O—C1-4 alkyl, wherein the C1-4 alkyl and —O—C1-4 alkyl are optionally further substituted with halogen, hydroxyl, methoxy, or ethoxy;
L is a linking group selected from the group consisting of:
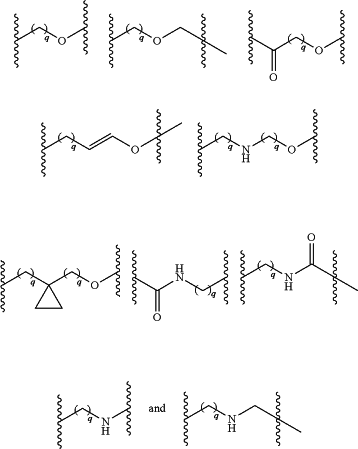 wherein each of the subscripts q is independently 1, 2, 3, or 4, and L is optionally further substituted with one or two members independently selected from the group consisting of halogen, hydroxy, C1-3 alkyl, —O—C1-3 alkyl, C1-3 hydroxyalkyl, C1-3 haloalkyl, and —CO2H;
Z is selected from the group consisting of azetidinyl and pyrrolidinyl, each of which is optionally substituted with from 1 to 4 groups independently selected from halogen, CN, hydroxy, oxo, C1-4 alkyl, —NH2, —NHC1-3alkyl, —N(C1-3alkyl)2, —O—C1-3 alkyl, C1-3 hydroxyalkyl, C1-3 haloalkyl, —OC(O)(C1-4 alkyl), —CO2(C1-4 alkyl), and —CO2H;
R2a is H or halogen;
each R2b and R2c is independently selected from the group consisting of H, halogen, —CN, —Rd, —CO2Re, —CONReRf, —OC(O)NReRf, —NRfC(O)Re, —NRfC(O)2Rd, —NRe—C(O)NReRf, —NReRf, —ORe, —X2—ORe, —X2—NReRf, —X2—CO2Re, —SF5, and —S(O)2NReRf, wherein each X2 is a C1-4 alkylene; each Re and Rf is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo; and each Rd is independently selected from the group consisting of C1-8 alkyl, C2-8 alkenyl, and C1-8 haloalkyl;
R3 is selected from the group consisting of —NRgRh and C4-12 heterocyclyl, wherein the C4-12 heterocyclyl is optionally substituted with 1 to 6 R3a;
each R3a is independently selected from the group consisting of halogen, —CN, oxo, —Ri, —CO2Rj, —CONRjRk, —CONHC1-6 alkyl-OH, —C(O)Rj, —OC(O)NRjRk, —NRjC(O)Rk, —NRjC(O)2Rk, —CONHOH, —PO3H2, —NRj—X3—C(O)2Rk, —NRjC(O)NRjRk, —NRjRk, —ORj, —S(O)2NRjRk, —O—X3—ORj, —O—X3—NRjRk, —O—X3—CO2Rj, —O—X3—CONRjRk, —X3—ORj, —X3—NRjRk, —X3—CO2Rj, —X3—CONRjRk, —X3—CONHSO2Rj, and SF5; wherein X3 is C1-6 alkylene and is optionally further substituted with OH, SO2NH2, CONH2, C(O)NHOH, PO3H2, COO—C1-8 alkyl, or CO2H, wherein each Rj and Rk is independently selected from hydrogen, C1-8 alkyl optionally substituted with 1 to 2 substituents independently selected from OH, SO2NH2, CONH2, C(O)NHOH, PO3H2, COO—C1-8alkyl, and CO2H, and C1-8 haloalkyl optionally substituted with 1 to 2 substituents independently selected from OH, SO2NH2, CONH2, C(O)NHOH, PO3H2, COO—C1-8alkyl, and CO2H, or when attached to the same nitrogen atom Rj and Rk can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo; and each Ri is independently selected from the group consisting of —OH, C1-8 alkyl, C2-8 alkenyl, and C1-8 haloalkyl, each of which may be optionally substituted with OH, SO2NH2, CONH2, C(O)NHOH, PO3H2, COO—C1-8 alkyl, or CO2H;
Rg is selected from the group consisting of H, C1-8 haloalkyl, and C1-8 alkyl;
Rh is selected from —C1-8 alkyl, C1-8 haloalkyl, C1-8 hydroxyalkyl, C1-8alkyl-CO2Rj, C1-8alkyl-CONRjRk, C1-8alkyl-CONHSO2Rj, C1-8 alkyl-SO2NRjRk, C1-8 alkyl-SO3Rj, C1-8 alkyl-B(OH)2, C1-8 alkyl-PO3H2, C1-8 alkyl-C(O)NHOH, C1-8 alkyl-NRh1Rh2, —C(O)Rj, C3-10 cycloalkyl, —C3-10 cycloalkyl-COORj, —C3-10 cycloalkyl-ORj, C4-8 heterocyclyl, —C4-8 heterocyclyl-COORj, —C4-8 heterocyclyl-ORj, —C1-8 alkyl-C4-8 heterocyclyl, —C(═O)OC1-8 alkyl-C4-8 heterocyclyl, —C1-8 alkyl-C3-10 cycloalkyl, C5-10 heteroaryl, —C1-8alkyl-C5-10 heteroaryl, —C1-8 alkyl-C6-10 aryl, —C1-8 alkyl-(C═O)—C6-10 aryl, —CO2—C1-8 alkyl-O2C—C1-8 alkyl, —C1-8 alkyl-NH(C═O)—C2-8 alkenyl, —C1-8 alkyl-NH(C═O)—C1-8 alkyl, —C1-8 alkyl-NH(C═O)—C2-8 alkynyl, —C1-8 alkyl-(C═O)—NH—C1-8 alkyl-COORj, and —C1-8 alkyl-(C═O)—NH—C1-8 alkyl-ORj optionally substituted with CO2H; or
Rh combined with the N to which it is attached is a mono-, di- or tri-peptide comprising 1-3 natural amino acids and 0-2 non-natural amino acids, wherein
the non-natural amino acids have an alpha carbon substituent selected from the group consisting of C2-4 hydroxyalkyl, C1-3 alkyl-guanidinyl, and C1-4 alkyl-heteroaryl,
the alpha carbon of each natural or non-natural amino acid is optionally further substituted with a methyl group, and
the terminal moiety of the mono-, di-, or tri-peptide is selected from the group consisting of C(O)OH, C(O)O—C1-6 alkyl, and PO3H2, wherein
Rh1 and Rh2 are each independently selected from the group consisting of H, C1-6 alkyl, and C1-4 hydroxyalkyl;
the C1-8 alkyl portions of Rh are optionally further substituted with from 1 to 3 substituents independently selected from OH, COOH, SO2NH2, CONH2, C(O)NHOH, COO—C1-8 alkyl, PO3H2, and C5-6 heteroaryl optionally substituted with 1 to 2 C1-3 alkyl substituents,
the C5-10 heteroaryl and the C6-10 aryl portions of Rh are optionally substituted with 1 to 3 substituents independently selected from OH, B(OH)2, COOH, SO2NH2, CONH2, C(O)NHOH, PO3H2, COO—C1-8alkyl, C1-4alkyl, C1-4alkyl-OH, C1-4alkyl-SO2NH2, C1-4alkyl CONH2, C1-4alkyl-C(O)NHOH, C1-4alkyl-PO3H2, C1-4alkyl-COOH, and phenyl, and the C4-8 heterocyclyl and C3-10 cycloalkyl portions of Rh are optionally substituted with 1 to 4 Rw substituents;
each Rw substituent is independently selected from C1-4 alkyl, C1-4 alkyl-OH, C1-4 alkyl-COOH, C1-4 alkyl-SO2NH2, C1-4 alkyl CONH2, C1-4 alkyl-C(O)NHOH, C1-4 alkyl-PO3H, OH, COO—C1-8 alkyl, COOH, SO2NH2, CONH2, C(O)NHOH, PO3H2, and oxo;
R4 is selected from the group consisting of O—C1-8 alkyl, —O—C1-4 alkyl-C6 aryl, —O—C1-4 alkyl-C4-7 heterocycloalkyl, and —O—C1-4 alkyl-C6 heteroaryl, each of which is optionally substituted with 1 or 2 R4a;
each R4a is independently selected from the group consisting of halogen, —CN, —Rm, —CONRnRp, and —ORn;
wherein each Rn and Rp is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl; and each Rm is independently selected from the group consisting of C1-8 alkyl, C2-8 alkenyl, and C1-8 haloalkyl;
n is 0, 1, 2, or 3;
each R5 is independently selected from the group consisting of halogen, —CN, —Rq, —CO2Rr, —CONRrRs, —C(O)Rr, —OC(O)NRrRs, —NRrC(O)Rs, —NRrC(O)2Rq, —NRr—C(O)NRrRs, —NRrRs, —ORr, —O—X5—ORr, —O—X5—NRrRs, —O—X5—CO2Rr, —O—X5—CONRrRs, —X5—ORr, —X5—NRrRs, —X5—CO2Rr, —X5—CONRrRs, —SF5, and —S(O)2NRrRs, wherein each X5 is a C1-4 alkylene; each Rr and Rs is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo; each Rq is independently selected from the group consisting of C1-8 alkyl and C1-8 haloalkyl;
R6a is selected from the group consisting of H, C1-4 alkyl, and C1-4 haloalkyl;
m is 0, 1, 2, 3, or 4; and
each R6b is independently selected from the group consisting of F, C1-4 alkyl, O—Ru, C1-4 haloalkyl, and NRuRv, wherein each Ru and Rv is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo.
|