| CPC A61K 31/497 (2013.01) [C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01)] | 19 Claims |
|
1. A compound of Formula (Ia), or a pharmaceutically acceptable salt, solvate, tautomer, or stereoisomer thereof:
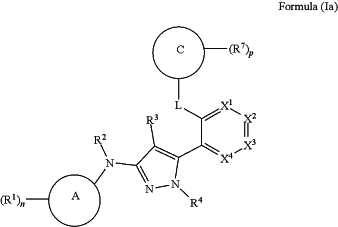 wherein:
Ring A is heteroaryl;
each R1 is independently deuterium, halogen, —CN, —NO2, —OH, —ORa, —OC(═O)Ra, —OC(═O)ORb, —OC(═O)NRcRd, —SH, —SRa, —S(═O)Ra, —S(═O)2Ra, —S(═O)2NRcRd, —NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra; —NRbC(═O)ORb, —NHS(═O)2Ra, —C(═O)Ra, —C(═O)ORb, —C(═O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl; or heteroaryl;
n is 0-4;
R2 is hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, or heterocycloalkyl;
R3 is hydrogen, deuterium, halogen, —CN, —NO2, —OH, —ORa; —NRcRd, —C(═O)Ra, —C(═O)ORb, —C(═O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, or heterocycloalkyl;
R4 is hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, cycloalkyl, or heterocycloalkyl;
X1 is N or CR5a;
X2 is N or CR5b;
X3 is N or CR5c;
X4 is N or CR5d;
R5a, R5b, R5c, and R5d are independently hydrogen, deuterium, halogen, —CN, —NO2, —ORa, —OC(═O)Ra, —OC(═O)ORb, —OC(═O)NRcRd, —SH, —SRa, —S(═O)Ra, —S(═O)2Ra, —S(═O)2NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(C═O)ORb, —NHS(═O)2Ra, —C(═O)ORb, —C(═O)NRcRd, —C(═O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
L is —O—;
Ring C is cycloalkyl;
each R7 is independently deuterium, halogen, —CN, —NO2, —OH, —ORa, —OC(═O)Ra, —OC(═O)ORb, —OC(═O)NRcRd, —SH, —SRa, —S(═O)Ra, —S(═O)2Ra, —S(═O)2NRcRd, —NRcRd, —NRbC(═O)NRcRd, —NRbC(═O)Ra, —NRbC(═O)ORb, —NHS(═O)2Ra, —C(═O)Ra, —C(═O)ORb, —C(═O)NRcRd, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C1-C6heteroalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
or two R7 on the same atom are taken together to form an oxo;
p is 1 or 2;
each Ra is independently C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C2-C6alkenyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more oxo, halogen, —CN, —OH, —OCH3, —S(═O)CH3, —S(═O)2CH3, —S(═O)2NH2, —S(═O)2NHCH3, —S(═O)2N(CH3)2, —NH2, —NHCH3, —N(CH3)2, —C(═O)CH3, —C(═O)OH, —C(═O)OCH3, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, or C1-C6aminoalkyl;
each Rb is independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more oxo, halogen, —CN, —OH, —OCH3, —S(═O)CH3, —S(═O)2CH3, —S(═O)2NH2, —S(═O)2NHCH3, —S(═O)2N(CH3)2, —NH2, —NHCH3, —N(CH3)2, —C(═O)OH, —C(═O)OCH3, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, or C1-C6aminoalkyl; and
each Rc and Rd are independently hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, C1-C6aminoalkyl, C2-C6alkenyl, C2-C6alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C1-C6alkyl(cycloalkyl), C1-C6alkyl(heterocycloalkyl), C1-C6alkyl(aryl), or C1-C6alkyl(heteroaryl); wherein each alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl is independently optionally substituted with one or more oxo, halogen, —CN, —OH, —OCH3, —S(═O)CH3, —S(═O)2CH3, —S(═O)2NH2, —S(═O)2NHCH3, —S(═O)2N(CH3)2, —NH2, —NHCH3, —N(CH3)2, —C(═O)CH3, —C(═O)OH, —C(═O)OCH3, C1-C6alkyl, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, or C1-C6aminoalkyl;
or Rc and Rd are taken together with the atom to which they are attached to form a heterocycloalkyl optionally substituted with one or more oxo, halogen, —CN, —OH, —OCH3, —S(═O)CH3, —S(═O)2CH3, —S(═O)2NH2, —S(═O)2NHCH3, —S(═O)2N(CH3)2, —NH2, —NHCH3, —N(CH3)2, —C(═O)CH3, —C(═O)OH, —C(═O)OCH3, C1-C6haloalkyl, C1-C6deuteroalkyl, C1-C6hydroxyalkyl, or C1-C6aminoalkyl.
|