| CPC A61B 90/92 (2016.02) [A61B 18/1206 (2013.01); A61B 18/14 (2013.01); A61B 34/25 (2016.02); A61B 90/94 (2016.02); A61B 18/02 (2013.01); A61B 2017/00119 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00809 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/00898 (2013.01); A61B 2018/00994 (2013.01); A61B 2018/1226 (2013.01)] | 31 Claims |
|
1. A system comprising:
an energy source for use with at least two treatment devices, the energy source being operable to be connected to the at least two treatment devices at a same time; and
an information display associated with the energy source, the information display being operable to provide information about the at least two treatment devices when the at least two treatment devices are connected to the energy source,
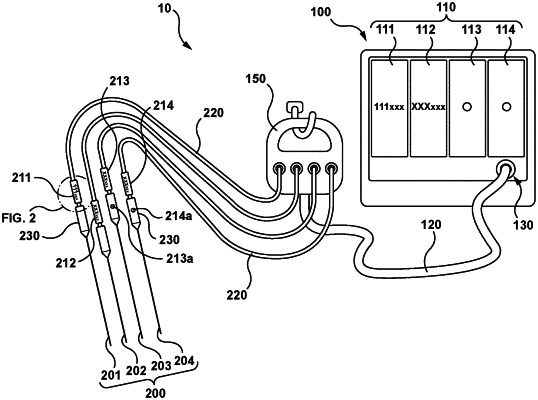
wherein the information display is configured to provide an association between a first device identifier associated with and connected to a first treatment device of the at least two treatment devices with first information provided on the information display about the first treatment device and an association between a second device identifier associated with and connected to a second treatment device of the at least two treatment devices with second information provided on the information display about the second treatment device, wherein both the first device identifier and the second device identifier are distinct from the information display and directly identifiable by a user, wherein the first device identifier comprises a first active visual identifier on the first treatment device, wherein the second device identifier comprises a second active visual identifier on the second treatment device, and wherein each of the first active visual identifier and the second active visual identifier is configured to indicate an operational status change of the respective first treatment device or second treatment device.
|