| CPC A61B 17/8685 (2013.01) [A61B 17/72 (2013.01); A61B 17/84 (2013.01); A61B 17/864 (2013.01); A61B 17/8802 (2013.01); A61B 17/8816 (2013.01); A61B 17/8819 (2013.01)] | 9 Claims |
|
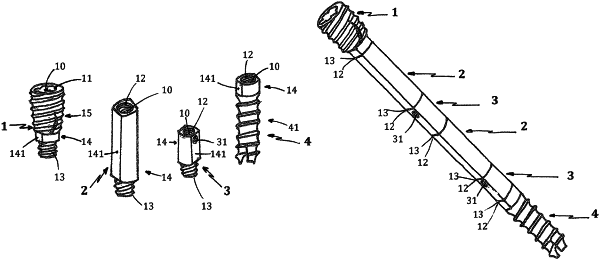
1. A modular medical implant allowing a targeted injection into a targeted injection site, the modular medical implant comprising a proximal module at one end, a distal module at another end, and one or several intermediate modules positioned between the proximal module and the distal module, wherein:
the proximal module comprises:
a first proximal module end,
a second proximal module end,
a proximal module length extending from the first proximal module end to the second proximal module end,
a proximal module body configured to receive a clamping tool between the first proximal module end and the second proximal module end,
a female imprint at the first proximal module end from which extends an inner lumen passing longitudinally through the proximal module length to the second proximal module end,
an exterior bone thread forming the first proximal module end extending along the proximal module length to the proximal module body, and
a male securing principle extending from the second proximal module end along the proximal module length to the proximal module body;
the one or several intermediate modules, of which one or several are perforated intermediate modules provided with perforations, each of the one or several intermediate modules comprises:
a first intermediate module end,
a second intermediate module end,
an intermediate module length extending from the first intermediate module end to the second intermediate module end,
an intermediate module body configured to receive a clamping tool extending from the first intermediate module end and towards the second intermediate module end
a female securing principle at the first intermediate module end from which extends an inner lumen passing longitudinally through the intermediate module length to the second intermediate module end, the perforations of the one or several perforated intermediate modules pass through from the lumen to a periphery of the intermediate module body, allowing for conveying of a liquid or pasty material to targeted locations, and
a male securing principle extending from the second intermediate module end to the intermediate module body; and
the distal module comprises:
a first distal module end,
a second distal module end,
a distal module length extending from the first distal module end to the second distal module end,
a distal module body configured to receive a clamping tool extending from the first distal module end and towards the second distal module end,
a female securing principle at the first distal module end from which extends an inner lumen passing longitudinally through the distal module length to the second distal module end,
an exterior bone thread extending from the distal module body along the distal module length to form the second distal module end,
wherein all of the modules required for producing a desired length of the modular medical implant, are secured to one another by an intermediary of cooperating sets comprising the male securing principles and the female securing principles, thus making it possible to position the perforations of the perforated intermediate module or modules at required levels to target injection of a liquid or pasty material, and
wherein the modular medical implant is configured to target a targeted injection site localized inside a bone cavity in order to carry out a filling and/or a consolidation of said cavity, while preventing any risk of constriction in the vicinity of the injection site.
|