| CPC C12Q 1/6874 (2013.01) [B05D 3/06 (2013.01); B05D 3/065 (2013.01); C09D 133/26 (2013.01); C09D 135/00 (2013.01); C12Q 1/6806 (2013.01); Y10T 428/24802 (2015.01); Y10T 428/24917 (2015.01); Y10T 428/24926 (2015.01); Y10T 428/2998 (2015.01); Y10T 428/31536 (2015.04); Y10T 428/31935 (2015.04)] | 20 Claims |
|
1. A substrate comprising a first plurality of oligonucleotides and a second plurality of oligonucleotides immobilized on a surface of the substrate through a polymer that is covalently attached to the surface of the substrate, wherein the polymer comprising a recurring unit of Formula (Ia) and a recurring unit of Formula (II):
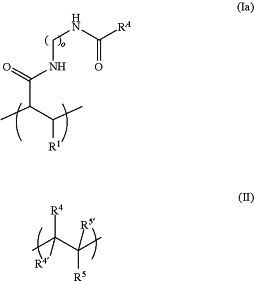 wherein:
R1 is H, alkyl, alkoxy, alkenyl, alkynyl, or optionally substituted variants thereof;
RA is azido;
each of the —(CH2)o— units is optionally substituted;
o is an integer between 1 and 50;
each R4, R4′, R5 and R5′ is independently selected from the group consisting of H, R6, OR6, —C(O)OR6, —C(O)R6, —OC(O)R6, —C(O)NR7R8, and —NR7R8;
each R6 is independently H, OH, alkyl, cycloalkyl, hydroxyalkyl, aryl, heteroaryl, or heterocyclyl, or an optionally substituted variant thereof; and
each R7 and R8 is independently H or alkyl, or R7 and R8 are joined together with the atom or atoms to which they are attached to form a heterocycle.
|