| CPC C07K 16/2863 (2013.01) [A61K 31/429 (2013.01); A61K 31/437 (2013.01); A61K 31/4745 (2013.01); A61K 31/4985 (2013.01); A61K 31/519 (2013.01); A61K 31/522 (2013.01); A61K 39/39558 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); A61K 38/16 (2013.01); A61K 2039/505 (2013.01); A61K 2039/585 (2013.01); A61K 2300/00 (2013.01); C07K 2317/24 (2013.01); C07K 2317/76 (2013.01)] | 12 Claims |
|
1. A combination, consisting of:
(i) a pharmaceutical composition consisting of an admixture of 1) an effective amount of an EGFR binding antagonist antibody, and 2) one or more pharmaceutically acceptable carriers, excipients or diluents;
(ii) a pharmaceutical composition consisting of an admixture of 1) an effective amount of an immunotherapeutic, and 2) one or more pharmaceutically acceptable carriers, excipients or diluents, wherein the immunotherapeutic is a compound of Formula (I):
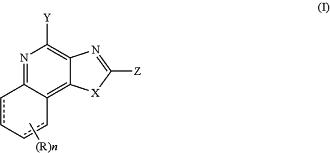 wherein dashed line represents bond or absence of bond;
X is S or —NR1, R1 is —W0—W1—W2—W3—W4,
W0 is a bond, alkyl, alkenyl, alkynyl, alkoxy, or -alkyl-S-alkyl-,
W1 is a bond, —O—, or —NR2—, wherein R2 is hydrogen, alkyl or alkenyl,
W2 is a bond, —O—, —C(O)—, —C(S)—, or —S(O)2—,
W3 is a bond, —NR3—, wherein R3 is hydrogen, alkyl or alkenyl,
W4 is hydrogen, alkyl, alkenyl, alkynyl, alkoxy, cycloalkyl, aryl, aryloxy, heteroaryl, or heterocyclyl, each of which is optionally substituted by one or more substituents selected from the group consisting of hydroxyl, alkoxy, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heteroaryl, heterocyclyl, —NH2, nitro, -alkyl-hydroxyl, -alkyl-aryl, -alkyl-heteroaryl, -alkyl-heterocyclyl, —O—R4, —O-alkyl-R4, -alkyl-O—R4, —C(O)—R4, -alkyl-C(O)—R4, -alkyl-C(O)—O—R4, —C(O)—O—R4, —S—R4, —S(O)2—R4, —NH—S(O)2—R4, -alkyl-S—R4, -alkyl-S(O)2—R4, —NHR4, —NR4R4, —NH-alkyl-R4, halogen, —CN, —NO2, and —SH, wherein R4 is independently hydrogen, alkyl, alkenyl, -alkyl-hydroxyl, aryl, heteroaryl, heterocyclyl, or haloalkyl;
Z is hydrogen, alkyl, alkenyl, alkynyl, alkoxy, aryl, haloalkyl, heteroaryl, heterocyclyl, each of which can be optionally substituted by one or more substituents selected from the group consisting of hydroxyl, alkoxy, alkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocyclyl, halogen, cyano, nitro, —N(R5)2, -alkoxy-alkyl, -alkoxy-alkenyl, —C(O)-alkyl, —C(O)—O-alkyl, —O—C(O)-alkyl, —C(O)—N(R5)2, aryl, heteroaryl, —CO-aryl, and —CO-heteroaryl, wherein each R5 is independently hydrogen, alkyl, haloalkyl, -alkyl-aryl, or -alkyl-heteroaryl;
R is hydrogen, alkyl, alkoxy, haloalkyl, halogen, aryl, heteroaryl, heterocyclyl, each of which is optionally substituted by one or more substituents selected from the group consisting of hydroxyl, alkoxy, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, heteroaryl, heterocyclyl, —NH2, nitro, -alkyl-hydroxyl, -alkyl-aryl, -alkyl-heteroaryl, -alkyl-heterocyclyl, —O—R4, —O-alkyl-R4, -alkyl-O—R4, —C(O)—R4, —C(O)—NH—R4, —C(O)—NR4R4, -alkyl-C(O)—R4, -alkyl-C(O)—O—R4, —C(O)—O—R4, —O—C(O)—R4, —S—R4, —C(O)—S—R4, —S—C(O)—R4, —S(O)2—R4, —NH—S(O)2—R4, -alkyl-S—R4, -alkyl-S(O)2—R4, —NHR4, —NR4R4, —NH-alkyl-R4 halogen, —CN, and —SH, wherein R4 is independently hydrogen, alkyl, alkenyl, alkoxy, -alkyl-hydroxyl, aryl, heteroaryl, heterocyclyl, or haloalkyl;
n is 0, 1, 2, 3, or 4;
Y is —NR6R7, —CR6R7R8, or -alkyl-NH2, each of which can be optionally substituted by one or more substituents selected from the group consisting of hydroxyl, alkoxy, alkyl, alkenyl, alkynyl, —NH2, halogen, —N(R5)2, -alkoxy-alkyl, -alkoxy-alkenyl, —C(O)-alkyl, —C(O)—O-alkyl, —C(O)—N(R5)2, aryl, heteroaryl, —CO-aryl, and —CO-heteroaryl,
wherein R6, R7 and R8 are independently hydrogen, alkyl, alkenyl, alkoxy, alkylamino, dialkylamino, alkylthio, arylthio, -alkyl-hydroxyl, -alkyl-C(O)—O—R9, -alkyl-C(O)—R9, or -alkyl-O—C(O)—R9, wherein each R5 is independently hydrogen, alkyl, haloalkyl, -alkyl-aryl, or -alkyl-heteroaryl, wherein R9 is hydrogen, alkyl, alkenyl, halogen, or haloalkyl;
X and Z taken together may optionally form a (5-9)-membered ring,
or a pharmaceutically acceptable salt or solvate thereof, that is capable of activating a human plasmacytoid dendritic cell, myeloid dendritic cell, or NK cell, or a combination thereof; and, optionally,
(iii) an effective amount of an additional therapeutic agent, wherein the additional therapeutic agent is an anticancer agent selected from the group consisting of a chemotherapeutic agent, an antimetabolite, an inhibitor of topoisomerase I and II, an alkylating agent, a microtubule inhibitor, an antiandrogen agent, a GNRh modulator and mixtures thereof;
wherein the EGFR binding antagonist antibody and the immunotherapeutic are not covalently linked.
|