| CPC C07K 16/2803 (2013.01) [A61K 47/6803 (2017.08); A61K 47/6867 (2017.08); A61P 35/02 (2018.01); C07D 487/04 (2013.01); C07K 2317/24 (2013.01); C07K 2317/53 (2013.01); Y02P 20/55 (2015.11)] | 10 Claims |
|
1. A conjugate of formula ConjE:
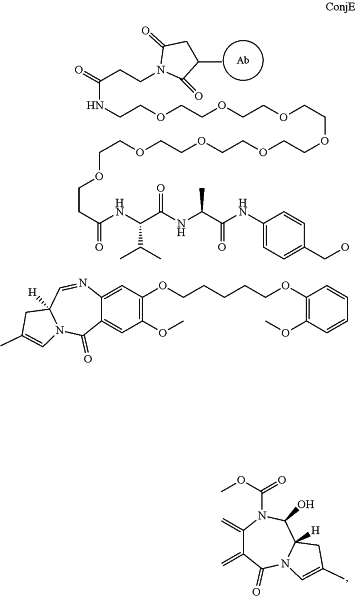 wherein Ab is an antibody which binds CD22 and comprises:
a VH domain having the amino acid sequence of SEQ ID NO. 1;
a VL domain having the amino acid sequence of SEQ ID NO. 2; and
heavy chains comprising an amino acid substitution of each of HC226 and HC229 according to the EU index as set forth in Kabat,
light chains each having an amino acid substitution of the interchain cysteine residue κLC214 or λLC213 according to the EU index as set forth in Kabat, and
heavy chains each retaining the unsubstituted interchain cysteine HC220 according to the EU index as set forth in Kabat, wherein the conjugation of the drug moiety to the antibody is at HC220 according to the EU index as set forth in Kabat.
|