| CPC C07D 498/04 (2013.01) [A61P 37/02 (2018.01)] | 24 Claims |
|
1. A method of treating a disease, disorder, or condition in a subject in need thereof, comprising administering an effective amount of a compound, or a pharmaceutically acceptable salt, solvate, or tautomer thereof, to the subject in need thereof,
wherein the disease, disorder, or condition is an inflammatory disease, disorder, or condition that is responsive to the inhibition of activation of the NLRP3 inflammasome,
and wherein the compound is of formula (Ib):
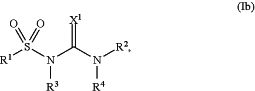 wherein:
X1 is O or S;
R1 is selected from the group consisting of
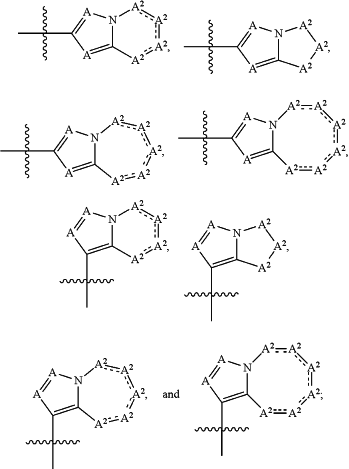  represents a single bond or a double bond provided that the ring comprising one or more A2 is a non-aromatic ring; represents a single bond or a double bond provided that the ring comprising one or more A2 is a non-aromatic ring;each A is independently CR5a N;
each A2 is independently CR5a, C(R5a)2, N, NR5a, O, S, or S(O)2;
R2 is
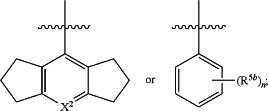 X2 is N or CR5b;
R3 and R4 are H;
each R5a is independently H, D, halogen, —OH, —CN, —NO2, —SR6, —OR6, —NHR6, —NR6R7, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C8cycloalkyl, heterocyclyl, aryl, heteroaryl, or —CH2-C3-C8cycloalkyl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, C2-C6alkynyl, C3-C8cycloalkyl, heterocyclyl, aryl, heteroaryl, and —CH2—C3-C8cycloalkyl are optionally substituted with D, halogen, C1-C6alkyl, —OR6, —NH2, —NH(C1-C6alkyl), or —N(C1-C6alkyl)2; or
two R5a together with the atoms to which they are attached can form C3-C8cycloalkyl or heterocyclyl; wherein the heterocyclyl contains 1-3 heteroatoms selected from the group consisting of N, S, P and O; wherein the C3-C8cycloalkyl and heterocyclyl are optionally substituted with D, halogen, C1-C6alkyl, —OR6, —NH2, —NH(C1-C6alkyl), or —N(C1-C6alkyl)2; or
two geminal R5a can form an oxo group;
each R5b is independently H, D, halogen, —OH, —CN, —NO2, —SR6, —OR6, —NHR6, —NR6R7, C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, or C2-C6alkynyl; wherein the C1-C6alkyl, C2-C6alkenyl, C4-C8cycloalkenyl, and C2-C6alkynyl are optionally substituted with D, halogen, —OR6, —NH2, —NH(C1-C6alkyl), or —N(C1-C6alkyl)2;
R6 and R7 are independently, at each occurrence, H, D, C1-C8alkyl, C2-C8alkenyl, C2-C8alkynyl, C3-C8cycloalkyl, C4-C8cycloalkenyl, heterocyclyl, aryl, or heteroaryl; wherein the heterocyclyl and heteroaryl contain 1-5 heteroatoms selected from the group consisting of N, S, P and O; wherein the C1-C6alkyl, C2-C8alkenyl, C2-C6alkynyl, C3-C8cycloalkyl, C4-C8cycloalkenyl, heterocyclyl, aryl, and heteroaryl are optionally substituted with D, halogen, C1-C6alkyl, —OH, —O—C1-C6alkyl, —NH2, —NH(C1-C6alkyl), or —N(C1-C6alkyl)2; or
R6 and R7 together with the atom to which they are attached can form heterocyclyl or heteroaryl containing 1-3 heteroatoms selected from the group consisting of N, S, P, and O; and
n is an integer from 0 to 5;
provided that when the ring comprising A is an imidazole, then at least one A2 is N, NR5a, O, S, or S(O)2.
|