| CPC C07D 211/58 (2013.01) [C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 409/12 (2013.01); C07D 409/14 (2013.01)] | 19 Claims |
|
1. A method for treating a disease mediated by HDAC1 and/or HDAC2 in a subject comprising administering to the subject a compound of Formula I:
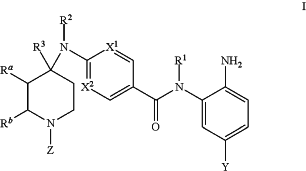 or a pharmaceutically acceptable salt thereof,
wherein,
X1 is CR7 or N;
X2 is CH or N;
wherein X1 and X2 are each N, or X1 and X2 are each CH;
Y is selected from the group consisting of:
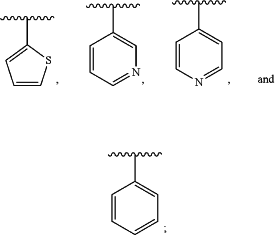 Z is selected from the group consisting of H, C1-C6-alkyl, C6-aryl, C(O)NR4R5, C(O)OR6, C(O)C1-C6-alkyl, C(O)C0-C6-alkyl-C6-aryl, C(O)—C3-C6-cycloalkyl, C(O)—C2-C6-heterocyclyl, and C(O)C0-6-alkyl-heteroaryl, wherein the aryl, heteroaryl, cycloalkyl, and heterocyclyl groups are optionally substituted by 1 or 2 of C1-C6-alkyl, halo, C1-C6-haloalkyl, hydroxy, or C1-C6-alkoxy;
Ra and Rb are H, or Ra and Rb together form a fused C6-aryl;
R1 is selected from the group consisting of H and C1-C6-alkyl;
R2 is selected from the group consisting of H, C1-C6-alkyl, and C6-aryl;
R3 is selected from the group consisting of H, C1-C6-alkyl, and C6-aryl;
or R2 and R3 together form a C2-C6-heterocyclyl;
R4 is selected from the group consisting of H, C1-C6-alkyl, C1-C6-alkyl-OH, and C1-C6—NH2;
R5 is C1-C6-alkyl;
or R4 and R5 together form a C2-C6-heterocyclyl, wherein heterocyclyl is optionally substituted by 1 or 2 of C1-C6-alkyl, halo, C1-C6-haloalkyl, hydroxy, or C1-C6-alkoxy;
R6 is selected from the group consisting of C1-C6-alkyl and C0-C6-alkyl-C6-aryl, wherein aryl is optionally substituted by 1 or 2 of C1-C6-alkyl, halo, or hydroxy; and
R7 is H;
wherein the disease mediated by HDAC1 and/or HDAC2 is selected from the group consisting of sickle-cell disease, beta-thalassemia, lung cancer, colon cancer, neuroblastoma, leukemia, and lymphoma.
|