| CPC C07D 211/44 (2013.01) [C07D 211/54 (2013.01); C07D 211/58 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 405/06 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 409/12 (2013.01); C07D 409/14 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01); C07D 471/02 (2013.01); C07D 471/04 (2013.01); C07D 471/08 (2013.01); C07D 491/048 (2013.01); C07D 495/02 (2013.01); C07D 495/04 (2013.01)] | 20 Claims |
|
1. A method of treating a subject who is suffering from a condition, disease, or disorder, wherein said condition, disease, or disorder is obesity, an eating disorder, cardiovascular disease, a gastrointestinal disorder, a dermatological disorder, metabolic disease, a viral disorder wherein FASN inhibition correlates inhibition of viral replication, cancer, or cancer metastasis, said method comprising administering to the subject a therapeutically effective amount of a compound according to Formula I:
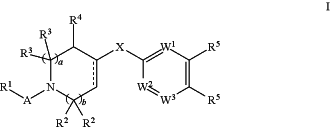 or a pharmaceutically acceptable salt thereof, wherein:
A is selected from —C(═O)— and —SO2—;
R1 is selected from —H, —(C1-C10) hydrocarbyl, substituted —(C1-C10) hydrocarbyl, 3-7 membered heterocyclyl, substituted 3-7 membered heterocyclyl, —(C6-C10) aryl, substituted (C6-C10) aryl, 5-6 membered heteroaryl, substituted 5-6 membered heteroaryl, —NR7R8, —OR7, —SR7, —N(OR8)R7, —N(SR8)R7 and —C(═O)—(C1-C6) alkyl;
a and b are independently selected from 0 and 1;
each R2 is independently selected from —H and —(C1-C4) alkyl;
each R3 is independently selected from —H and —(C1-C4) alkyl
R4 is selected from —H, —(C1-C6) alkyl, ═O, —OH, —O(C1-C6) alkyl, halogen, and —CN; wherein one of the R3 groups can optionally be structurally connected to one of the R2 groups to form an alkylene bridge to produce a bicyclic ring; or
one of the R3 groups can optionally be structurally connected to the R1 group to form a 5 to 7 membered heterocyclyl ring fused to the 1-2 face of the piperidine ring; or
one of the R3 groups can optionally be structurally connected to the R4 group to form a 5-7 membered carbocyclic or heterocyclic ring fused to the 2-3 face of the piperidine ring;
 indicates that the designated bond is a carbon-carbon single bond or a carbon-carbon double bond; indicates that the designated bond is a carbon-carbon single bond or a carbon-carbon double bond;X is selected from —O—, —S—, —SO—, —SO2—, —NH— and —NR9—;
W1, W2 and W3 are independently selected from N, CH, and C—R10; provided that W2 and W3 are not both N;
R5 is selected from —H, —C1-C7 hydrocarbyl, —C3-C6 heterocyclyl; halogen, —(C1-C3) haloalkyl, —O—(C1-C7) hydrocarbyl, —O— substituted —(C1-C7) hydrocarbyl, —O—C(═O)R8, —O(C1-C6) heteroalkyl, —CN, —NR7aR8a, —O(CH2)nNR7aR8a, —O(CH2)nOR8a, —NR8a(CH2)nNR7aR8a, —NR8a(CH2)nOR8a, —C(═O)NR7aR8a, —C(═O)OR7a, 5-6 membered heteroaryl, substituted 5-6 membered heteroaryl, 8-10 membered bicyclic heteroaryl, and substituted 8-10 membered bicyclic heteroaryl;
n is an integer selected from 1, 2, 3, and 4;
R6 is selected from:
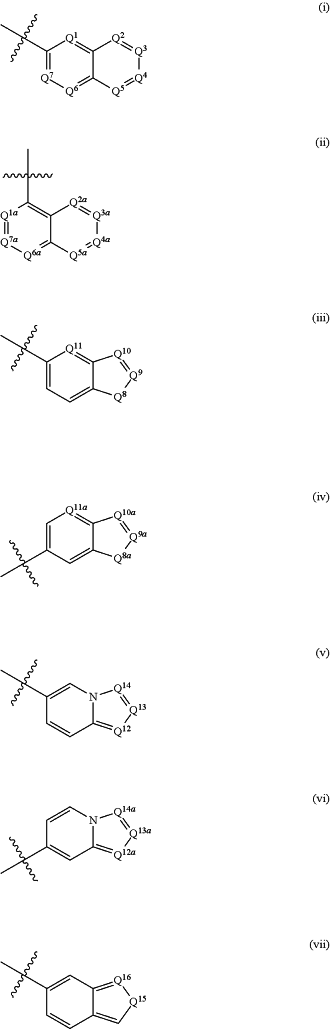 wherein, when R6 is (i), Q1, Q2, Q3, Q4, Q5, Q6 and Q7 are independently selected from N and C—R12, provided that 1, 2 or 3 of Q1, Q2, Q3, Q4, Q5, Q6 and Q7 are N, and the remainder of Q1, Q2, Q3, Q4, Q5, Q6 and Q7 are C—R12;
when R6 is (ii), Q1a, Q2a, Q3a, Q4a, Q5a, Q6a and Q7a are independently selected from N and C—R12, provided that 1, 2 or 3 of Q1a, Q2a, Q3a, Q4a, Q5a Q6a and Q7a are N, and the remainder of Q1a, Q2a, Q3a, Q4a, Q5a, Q6a and Q7a are C—R12;
when R6 is (iii), Q8 is selected from O, S, and N—R12n, Q9, Q10 and Q11 are independently selected from N and C—R12, provided that 1 or 2 of Q9, Q10 and Q11 are N, and the remainder of Q9, Q10 and Q11 are C—R12;
when R6 is (iv), Q8a is selected from O, S, and N—R12n, Q9a, Q10a and Q11a are independently selected from N and C—R12, provided that 1 or 2 of Q9a, Q10a and Q11a are N, and the remainder of Q9a, Q10a and Q11a are C—R12;
when R6 is (v), Q12, Q13 and Q14 are independently selected from N and C—R12; and
when R6 is (vi), Q12a, Q13a and Q14a are independently selected from N and C—R12;
when R6 is (vii), Q15 is selected from N—R12n and C—R12 and Q16 is selected from N and C—R12; provided that Q15 and Q16 are not both C—R12;
wherein the non-bridgehead ring carbon ring atoms in (i), (ii), (iii), (iv), (v), (vi) and (vii) are substituted or unsubstituted;
and wherein each R12 is independently selected from —H halogen, —(C1-C6) alkyl, —(C3-C6) cycloalkyl, 5-6 membered heterocyclyl, —OH, —O(C1-C6) alkyl, —O(CH2)r-(5-6 membered heterocycyl), —O(CH2)r—O(C1-C6) alkyl, —NH2, —CN, —NH(C1-C6) alkyl, —N(C1-C6 alkyl)2, —NH(CH2)r—O(C1-C6) alkyl, —NH(CH2)r—N(C1-C6 alkyl)2, —C(═O)NH2, —C(═O)NH(C1-C6) alkyl, and —C(═O)N(C1-C6 alkyl)2; wherein r is an integer selected independently from 1, 2, 3, and 4; and
each R12n is independently selected from —H, —(C1-C7) hydrocarbyl and substituted (C1-C7) hydrocarbyl;
R7 is selected from —H, —(C1-C7) hydrocarbyl, substituted —(C1-C7) hydrocarbyl, —C(═O)R8, —(C1-C6) heteroalkyl, 5-6 membered aryl, 5-6 membered heteroaryl and 5-6 membered heterocycyl;
R8 is selected from —H, and —(C1-C6) alkyl, wherein R7 can optionally be structurally connected to R8 to form a 5 to 7 membered heterocycyl ring;
R7a is selected from —H, —(C1-C7) hydrocarbyl, substituted —(C1-C7) hydrocarbyl, —C(═O)R8, and —(C1-C6) heteroalkyl;
R8a is selected from —H, and —(C1-C6) alkyl, wherein R7a can optionally be structurally connected to R8a to form a 5 to 7 membered heterocycyl ring;
R9 is selected from —(C1-C7) hydrocarbyl, wherein R9 can optionally be structurally connected to R4 to form a 5 to 7 membered heterocycyl ring;
each R10 is independently selected from —(C1-C7) hydrocarbyl, substituted —(C1-C7) hydrocarbyl, halogen, —C(═O)(C1-C7) hydrocarbyl, —C(═O)NH2, —C(═O)NH(C1-C7) hydrocarbyl, —C(═O)N((C1-C7)hydrocarbyl)2, —O(C1-C7) hydrocarbyl, substituted —O(C1-C7) hydrocarbyl, —(C3-C6) heterocycyl, substituted —(C3-C6)heterocyclyl-CN, —NH2, —NH(C1-C6)alkyl, —N(C1-C6 alkyl)2, —NH(CH2)m—R11, —N(C1-C6 alkyl)(CH2)m—R11, —O—(CH2)m—R11, and —(C1-C6) heteroalkyl;
m is an integer independently selected from 1, 2, 3, and 4; and
R11 is selected from —O(C1-C6)alkyl, —N(C1-C6 alkyl)2, —(C3-C6)heterocyclyl and substituted —(C3-C6) heterocycyl.
|