| CPC A61K 35/17 (2013.01) [A61K 39/3955 (2013.01); A61P 35/00 (2018.01); C07K 16/2851 (2013.01); C07K 2317/73 (2013.01)] | 7 Claims |
|
5. A composition comprising:
a) an immune cell expressing a CAR comprising:
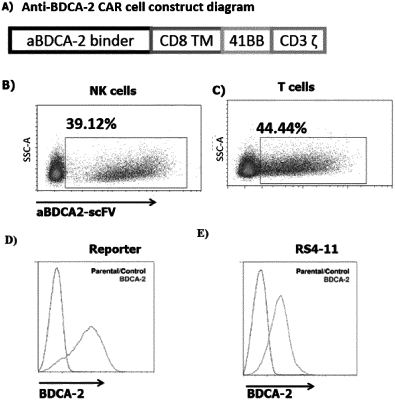
i) an antigen binding domain specific for a tag of a tagged polypeptide, wherein said tagged polypeptide is an antibody or antigen binding fragment thereof comprising the amino acid sequence of SEQ ID NO: 1 and SEQ ID NO: 2,
ii) a transmembrane domain,
iii) an intracellular signaling domain, and
wherein said antigen binding domain specifically binds a tag of a tagged polypeptide, wherein said polypeptide binds specifically to the antigen BDCA2 expressed on the surface of a target cell, wherein said target cell is a cancer cell expressing BDCA2, and
b) said tagged polypeptide.
|