| CPC A61K 31/551 (2013.01) [A61K 9/0053 (2013.01); A61K 31/4545 (2013.01); A61K 31/4725 (2013.01); A61K 45/06 (2013.01)] | 7 Claims |
|
1. A method for treating a patient having a neurological condition, the method comprising:
orally administering a pharmaceutical formulation comprising therapeutically effective amount of a rho kinase inhibitor to the patient, wherein the rho kinase inhibitor is Compound 1, having the structure:
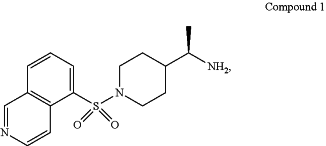 or a pharmaceutically acceptable salt thereof, wherein administering the pharmaceutical formulation to the patient results in a reduction of one or more symptoms of the neurological condition, and
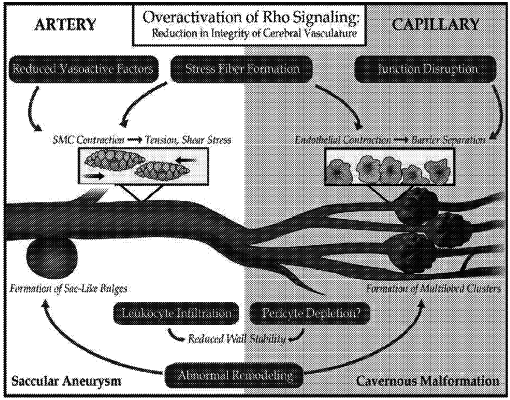
wherein the neurological condition is any of cerebral cavernous malformation, cavernous angioma, stroke, cerebral aneurysm, vasospasm, subarachnoid hemorrhage, cavernoma, a mutation in CCM1, a mutation in CCM2, a mutation in CCM3, or combinations thereof.
|