| CPC A61K 31/485 (2013.01) [A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2027 (2013.01); A61K 9/2031 (2013.01); A61K 9/2054 (2013.01); A61K 9/2826 (2013.01); A61K 9/2853 (2013.01)] | 13 Claims |
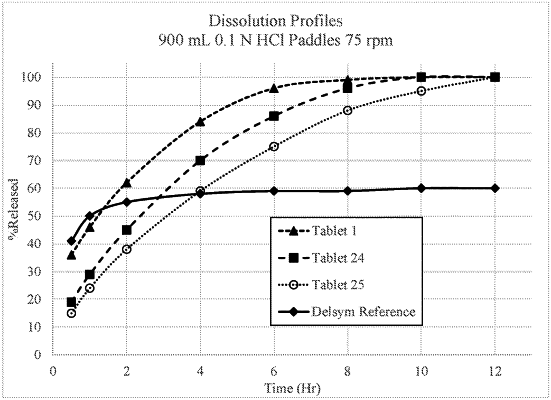
|
1. A sustained release pharmaceutical tablet composition comprising:
a) a core tablet sustained release formulation comprising
i. dextromethorphan HBr in an amount selected from about 12.50%, 17.44%, 18.40%, 23.26%, 23.28%, 23.37%, 23.50%, 23.83%, 25.00%, 25.75%, 26.02%, 26.67%, and 28.17% by weight of the composition;
ii. hydroxypropyl methylcellulose having an apparent viscosity of from about 2,663 to about 4,970 mPa·s at 2 wt % in water, a methyl substitution between about 22.0% and 24.0%, and a hydroxypropyl substitution between about 7.5% and 9.5%, in an amount selected from the group consisting of about 8.33%, 8.58%, 8.89%, 9.12%, 9.13%, 9.17%, 9.22%, 9.29%, 9.39%, 9.52%, 12.27%, 14.08%, 15.89%, 16.67%, 17.17%, 17.44%, 17.78%, 18.40%, 18.78%, 22.39%, 25.00%, 27.50%, 33.33%, and 33.91% by weight of the composition;
iii. hydroxypropyl methylcellulose having an apparent viscosity of from about 13,275 to about 24,780 mPa·s, a methyl substitution between about 22.0% and 24.0%, and a hydroxypropyl substitution between about 8.5% and 10.5%, in an amount selected from the group consisting of about 8.33%, 9.12%, 9.13%, 9.17%, 9.29%, 9.39%, 9.52%, 12.22%, 12.50%, 12.88%, 13.33%, 14.08%, 15.67%, 15.89%, 16.67%, 17.17%, 17.44%, 17.78%, 18.40%, 18.78%, and 23.01% by weight of the composition;
iv. lactose monohydrate in an amount selected from about 14.89%, 17.24%, 17.30%, 17.82%, 18.45%, 19.49%, 19.60%, 22.25%, 22.64%, 23.25%, 23.55%, 23.75%, 23.76%, 24.00%, 24.67%, 25.02%, 25.41%, 25.87%, 26.23%, 26.32%, 27.73%, 27.80%, 29.73%, 29.74%, 29.86%, 29.99%, 30.28%, 30.60%, and 40.67% by weight of the composition;
v. microcrystalline cellulose in an amount selected from about 0%, 13.53%, 15.46%, 15.89%, 16.67%, 17.22%, 17.39%, 18.14%, 18.22%, 19.24%, 19.31%, 19.60%, 20.21%, 20.34%, 20.65%, 20.67%, 20.75%, 20.84%, 20.95%, 21.04%, 21.26%, 21.70%, 23.25%, 23.61%, 25.47%, 26.23%, 26.59%, and 30.37% by weight of the composition;
vi. fumed silica in an amount selected from about 0.33%, 0.35%, 0.45%, 0.46%, 0.47%, 0.48%, 0.49%, or 0.50% by weight of the composition; and
vii. magnesium stearate in an amount selected from about 0.35%, 0.49%, 0.50%, 0.67%, 0.69%, 0.70%, 0.71%, 0.72%, 0.73%, and 0.75% by weight of the composition;
b) optionally, an active coating comprising:
i. dextromethorphan or a pharmaceutically acceptable salt thereof in an amount selected from about 1.86%, 4.11%, 4.12%, 4.15%, and 4.21% by weight of the composition;
ii. hydroxypropyl methylcellulose having an apparent viscosity range of about 2.4-7 mPa·s at 2 wt % in water, a methyl substitution range of about 28.0%-30.0%, and a hydroxypropyl substitution range of about 7.0%-12.0% in an amount selected from about 0%, 0.41%, 0.47%, and 0.91% by weight of the composition;
iii. polyvinyl pyrrolidone in an amount selected from about 0%, 0.41%, 0.91%, 1.37%, and 1.38% by weight of the composition;
iv. sodium lauryl sulfate in an amount selected from about 0%, 0.004%, 0.01%, and 0.06% by weight of the composition;
v. PEG 400 or PEG 8000 in an amount selected from about 0%, 0.20%, and 0.46% by weight of the composition;
and c) the film coating is absent or comprises a polymer and a plasticizer;
wherein the tablet has a volume of from about 0.0063 in3 to about 0.0183 in3, a surface area of from about 0.194 in2 to about 0.395 in2, and a surface area to volume ratio of from about 19.4 in−1 to about 31.0 in−1.
|