| CPC A61K 31/137 (2013.01) [A61K 8/41 (2013.01); A61K 8/44 (2013.01); A61K 8/49 (2013.01); A61K 8/4926 (2013.01); A61K 31/13 (2013.01); A61K 31/197 (2013.01); A61K 31/423 (2013.01); A61K 31/435 (2013.01); A61K 31/438 (2013.01); A61K 31/47 (2013.01); A61Q 19/00 (2013.01); A61Q 19/08 (2013.01); C08K 5/04 (2013.01); C08K 5/08 (2013.01); C08K 5/18 (2013.01)] | 41 Claims |
|
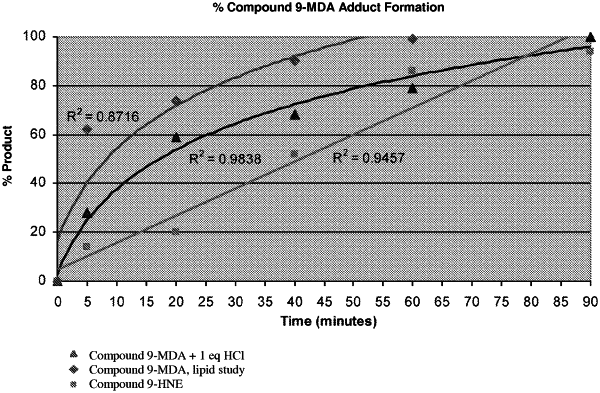
1. A method of treating scleritis, comprising:
administering to a subject in need thereof a therapeutically effective amount of a compound of formula (I):
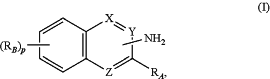 or a pharmaceutically acceptable salt thereof, wherein,
X is CH; Z is N; and Y is C with the —NH2 attached;
p is 0, 1, 2, or 3;
each RB is independently a halogen, hydroxyl, carbamoyl, amino, or aryl;
RA is
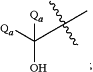 and
each Qa is independently a C1-C6 straight chain alkyl.
|