| CPC A61B 5/053 (2013.01) [A61B 5/6853 (2013.01); A61B 18/1492 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00875 (2013.01); A61B 2090/065 (2016.02)] | 20 Claims |
|
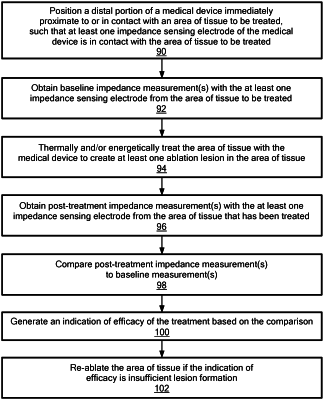
1. A method of assessing lesion formation, the method comprising:
recording a baseline impedance measurement from an area of tissue with a medical device;
ablating the area of tissue with the medical device;
recording a post-treatment impedance measurement from the area of tissue with the medical device after completion of ablating the area of tissue;
identifying at least one amplitude characteristic of the baseline impedance measurement and identifying at least one amplitude characteristic of the post-treatment impedance measurement;
comparing the at least one amplitude characteristic of the baseline impedance measurement and the at least one amplitude characteristic of the post-treatment impedance measurement;
generating an indication of efficacy based on the comparison and providing the indication of efficacy to a display, the indication of efficacy being one of sufficient lesion formation and insufficient lesion formation; and
re-ablating the area of tissue if the indication of efficacy is insufficient lesion formation.
|