| CPC H01M 12/08 (2013.01) [H01M 4/42 (2013.01); H01M 4/587 (2013.01); H01M 4/628 (2013.01); H01M 50/411 (2021.01); H01M 50/44 (2021.01); H01M 50/46 (2021.01); H01M 50/586 (2021.01)] | 19 Claims |
|
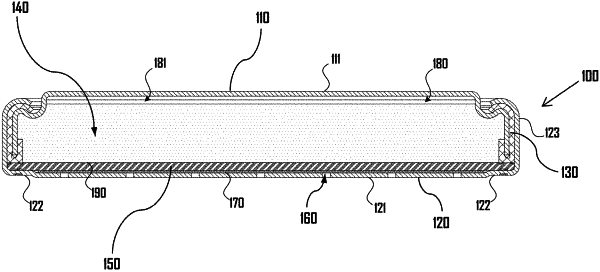
1. A zinc-air battery cell assembly comprising:
a metal cathode can that includes:
a planar base,
an elongated cathode sidewall that extends approximately perpendicular to a plane of the planar base, the elongated cathode sidewall extending to a terminal cathode sidewall end, and
a plurality of air holes defined by the planar base;
a metal anode can that includes:
a planar top end,
an elongated anode sidewall that extends approximately perpendicular to a plane of the planar top end, the elongated anode sidewall extending to a terminal anode sidewall end, the metal anode can disposed nested within the metal cathode can with the elongated anode sidewall disposed parallel and adjacent to the elongated cathode sidewall;
a cavity defined by the metal cathode can and the metal anode disposed nested within the metal cathode can,
a set of materials disposed within the cavity, the set of materials including:
a layer of anode material that includes:
zinc at 62-74% Weight %,
an aqueous electrolyte comprising by weight, 30-40% potassium hydroxide, 1%-5% Zinc Oxide,
one or more gelling agents in an aqueous slurry, and
one or more corrosion inhibitors, including 18 Crown 6, that causes the layer of anode material to have a gassing rate less than or equal to 0.5 cm3 after 1 week at 60° C.,
a void volume directly between and defined by the layer of anode material and the planar top end of the metal anode can,
a layer of cathode material that includes:
a carbon-polymer composite;
metal oxides that promote an oxygen reduction reaction;
a carbon-covered nickel mesh embedded within the composite and fixed to an internal face of the metal cathode can with either pressure or a conductive glue, the carbon-covered nickel mesh defined by a plurality of elongated grid elements disposed in a plurality of parallel rows and parallel columns, with the rows and columns being perpendicular to each other and engaging at a plurality of intersections,
a separator directly between and engaging both the layer of anode material and the layer of cathode material that acts as both an electronic insulator and an ion conductive path between the layer of anode material and the layer of cathode material, the separator including polyvinyl alcohol (PVA) fibers blended with cellulose and
a conductive diffusion member directly between and engaging both the planar base of the metal cathode can and the layer of cathode material; and
a grommet that provides a seal between the metal cathode can and the metal anode can while also keeping the metal anode can and metal cathode can physically and electrically separate.
|