| CPC C07K 14/805 (2013.01) [A61P 1/16 (2018.01); A61P 7/08 (2018.01); A61P 9/10 (2018.01); A61P 13/12 (2018.01); A61P 25/00 (2018.01); A61P 35/00 (2018.01); C07K 1/1077 (2013.01); A61K 38/00 (2013.01)] | 1 Claim |
|
1. A method of supplying oxygen to the tissues and organs in a subject in need thereof wherein the method comprises transfusing into the system of the subject a therapeutically effective amount of a thiosucciniyl-crosslinked hemoglobin comprising a tetrameric hemoglohin and at least one thiosuccinyl crosslinking moiety of Formula 1:
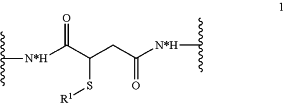 or a pharmaceutically acceptable salt or zwitterion thereof, wherein
each N* independently represents a nitrogen selected from the group consisting of a nitrogen in a lysine residue side chain in the tetrameric hemoglobin and a nitrogen at a N-terminus in the tetrameric hemoglobin; and
R1 is alkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, aralkyl, heteroaryl, or —(CR2)nY, wherein n is an integer selected from 0-10; R for each instance is independently hydrogen, alkyl, aralkyl, alkenyl, cycloalkyl, heterocycloalkyl, aryl, or heteroaryl; or two instances of R taken together form a 3-6 membered cycloalkyl or heterocycloalkyl containing 1, 2, or 3 heteroatoms selected from N, O, and S; and Y is selected from the group consisting of OR4, SR4, N(R4)2, —(C═O)R4, —(C═O)OR4, —O(C═O)R4, —O(C═O)OR4, —(C═O)N(R4)2, —(NR4)(C═O)R4, —(NR4)(C═O)OR4, —O(C═O)N(R4)2, —O(C≡NR4)N(R4)2, —(NR4)(C═O)N(R4)2, —(C≡NR4)N(R4)2, —(NR4)(C≡NR4)N(R4)2, —(S═O)R4, —S(O)2R4, —S(O)2OR4, —S(O)2N(R4)2, —OS(O)2R4, —(NR4)S(O)2R4, —OS(O)2OR4, —OS(O)2N(R4)2, —(NR4)S(O)2N(R4)2, —(NR4)S(O)2OR4, and —(CRR2R3), wherein R2 is hydrogen, alkyl, aralkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, OR4, SR4, N(R4)2, —(C═O)R4, —(C═O)OR4, —O(C═O)R4, —O(C═O)OR4, —(C═O)N(R4)2, —(NR4)(C═O)R4, —(NR4)(C═O)OR4, —O(C═O)N(R4)2, —O(C≡NR4)N(R4)2, —(NR4)(C═O)N(R4)2, —(C≡NR4)N(R4)2, —(NR4)(C≡NR4)N(R4)2, —(S═O)R4, —S(O)2R4, —S(O)2OR4, —S(O)2N(R4), —OS(O)2R4, —(NR4)S(O)2R4, —OS(O)2OR4, —OS(O)2N(R4)2, —(NR4)S(O)2N(R4)2, or —(NR4)S(O)2OR4; R3 is hydrogen, alkyl, aralkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, OR4, SR4, N(R4)2, —(C═O)R4, —(C═O)OR4, —O(C═O)R4, —O(C═O)OR4, —(C═O)N(R4)2, —(NR4)(C═O)R4, —(NR4)(C═O)OR4, —O(C═O)N(R4)2, —O(C≡NR4)N(R4)2, —(NR4)(C═O)N(R4)2, —(C≡NR4)N(R4)2, —(NR4)(C≡NR4)N(R4)2, —(S═O)R4, —S(O)2R4, —S(O)2OR4, —S(O)2N(R4)2, —OS(O)2R4, —(NR4)S(O)2R4, —OS(O)2OR4, —OS(O)2N(R4)2, —(NR4)S(O)2N(R4)2, or —(NR4)S(O)2OR4; and R4 for each instance is independently selected from the group consisting of hydrogen, alkyl, aralkyl, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; or R1 is a moiety selected from the group consisting of:
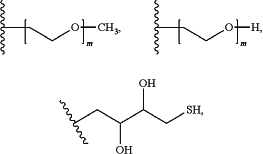 N5-(1-((carboxymethyl)amino)-1-oxo-3λ3-propan-2-yl)glutamine or a pharmaceutically acceptable salt thereof wherein m is a whole number selected from 1-1000, and wherein the thiosuccinyl-crosslinked hemoglobin is substantially pure.
|