| CPC C07D 498/08 (2013.01) [A61P 31/04 (2018.01); A61P 31/10 (2018.01); A61P 31/12 (2018.01); A61P 35/00 (2018.01); A61P 37/02 (2018.01)] | 20 Claims |
|
1. A method of assaying a compound for PD-1/PD-L1 interaction activity, said method comprising
(a) affixing human PD-L1 to a solid surface;
(b) contacting the compound with the affixed human PD-L1 to form an interaction mixture;
(c) contacting the interaction mixture with human PD-1 complexed with biotin to form a competition mixture;
(d) washing the competition mixture with a wash buffer to form an incubation product;
(e) contacting the incubation product with Streptavidin-HRP to form a signal mixture;
(f) washing the signal mixture with a wash buffer to form an detection product;
(g) contacting the detection product with 3,3′,5,5′-Tetramethylbenzidine (TMB) to form a signal product; and
(h) measuring the absorbance of the signal product at 450 nm,
wherein said method comprises performing steps (a)-(h) with a positive control sample having a formula represented by the structure
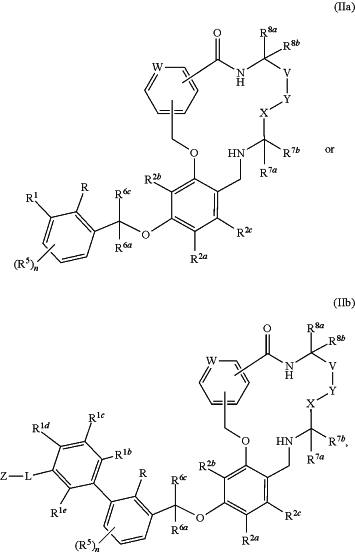 or a pharmaceutically acceptable salt thereof, wherein:
W is N or C(R9);
X, Y, and V are each independently selected from the group consisting of a bond, O, NH, N(CH3), C(O), methylene, and ethylene, wherein the methylene and ethylene are optionally substituted with one or two R7a;
R is selected from the group consisting of H, halogen, CN, C1-3 haloalkyl, C1-3 alkyl, and C1-3 alkoxy;
R1 is phenyl, optionally substituted with 1 to 3 R1a substituents;
each R1a is independently selected from the group consisting of halogen, —CN, —Rc, —CO2Ra, —CONRaRb, —C(O)Ra, —OC(O)NRaRb, —NRbC(O)Ra, —NRbC(O)2Rc, —NRa—C(O)NRaRb, —NRaRb, —ORa, —O—X1—ORa, —O—X1—CO2Ra, —O—X1—CONRaRb, —X1—ORa, —X1—NRaRb, —X1—CO2Ra, —X1—CONRaRb, —SF5, and —S(O)2NRaRb, wherein each X1 is a C1-4 alkylene; each Ra and Rb is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, wherein the five or six-membered ring is optionally substituted with oxo; each Rc is independently selected from the group consisting of C1-8 alkyl, C2-8 alkenyl, C2-8 alkynyl, and C1-8 haloalkyl; and optionally when two R1a substituents are on adjacent atoms, they are combined to form a fused five, six, or seven-membered carbocyclic or heterocyclic ring optionally substituted with from 1 to 3 substituents independently selected from halogen, oxo, C1-8 haloalkyl, and C1-8 alkyl;
each of R1b, R1c, R1d, and R1e is independently selected from the group consisting of H, halogen, CF3, CN, C1-4 alkyl, and —O—C1-4 alkyl, wherein the C1-4 alkyl and —O—C1-4 alkyl are optionally further substituted with halogen, hydroxyl, methoxy, or ethoxy;
L is a linking group selected from the group consisting of:
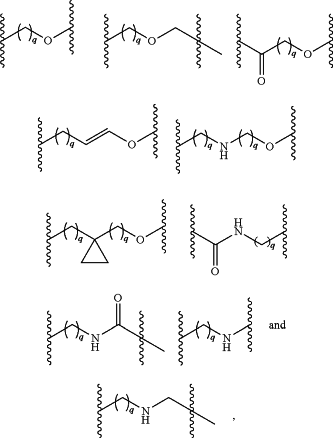 wherein each of the subscripts q is independently 1, 2, 3, or 4, and L is optionally further substituted with one or two members selected from the group consisting of halogen, hydroxy, C1-3 alkyl, —O—C1-3 alkyl, C1-3 hydroxyalkyl, C1-3 haloalkyl, and —CO2H;
Z is selected from the group consisting of azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, pyridyl, pyrimidinyl, guanidinyl, quinuclidine, and 8-azabicyclo[3.2.1]octane, each of which is optionally substituted with from 1 to 3 groups independently selected from halogen, hydroxy, C1-3 alkyl, —NH2, —NHC1-3alkyl, —N(C1-3alkyl)2, —O—C1-3 alkyl, C1-3 hydroxyalkyl, C1-3 haloalkyl, and —CO2H;
each R2a, R2b, and R2c is independently selected from the group consisting of H, halogen, —CN, —Rd, —CO2Re, —CONReRf, —OC(O)NReRf, —NRfC(O)Re, —NRfC(O)2Rd, —NRe—C(O)NReRf, —NReRf, —ORe, —X2—ORe, —X2—NReRf, —X2—CO2Re, —SF5, and —S(O)2NReRf, wherein each X2 is a C1-4 alkylene; each Re and Rf is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo; and each Rd is independently selected from the group consisting of C1-8 alkyl, C2-8 alkenyl, and C1-8 haloalkyl;
each R5 is independently selected from the group consisting of halogen, —CN, —Rq, —CO2Rr, —CONRrRs, —C(O)Rr, —OC(O)NRrRs, —NRrC(O)Rs, —NRrC(O)2Rq, —NRr—C(O)NRrRs, —NRrRs, —ORr, —O—X5—ORr, —O—X5—NRrRs, —O—X5—CO2Rr, —O—X5—CONRrRs, —X5—ORr, —X5—NRrRs, —X5—CO2Rr, —X5—CONRrRs, —SF5, and —S(O)2NRrRs, wherein each X5 is a C1-4 alkylene; each Rr and Rs is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring having from 0 to 2 additional heteroatoms as ring members selected from N, O, and S, and optionally substituted with oxo; and each Rq is independently selected from the group consisting of C1-8 alkyl and C1-8 haloalkyl;
R6a and R6c are each independently selected from the group consisting of H, C1-4 alkyl, and C1-4 haloalkyl;
each R7a and R7b is independently selected from the group consisting of H, C1-6 alkyl, CO2H, CH2OH, —CO2—(C1-6alkyl), and PO3H2, wherein C1-6 alkyl is optionally substituted with one or two members selected from halogen, OH, NH2, CN, and CO2H;
each R8a and R8b is independently selected from the group consisting of H and C1-6 alkyl, optionally substituted with halogen, OH, NH2, CN, and CO2H;
R9 is selected from the group consisting of H, halogen, CN, C1-6 alkyl, —O—C1-6 alkyl, —SO2(C1-6 alkyl), —C1-6 alkyl-CO2H, —C1-6 alkyl-CO2—C1-6 alkyl, —C1-6 alkyl-C(O)NH2, —C1-6 alkyl-C(O)NHC1-6 alkyl, and —C1-6 alkyl-C(O)N(C1-6 alkyl)2; and
n is 0, 1, 2, or 3.
|