| CPC C07D 401/14 (2013.01) [C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 417/14 (2013.01)] | 14 Claims |
|
1. A compound of formula (II):
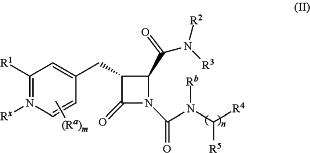 or a pharmaceutically acceptable salt thereof, wherein
R1 is hydrogen or —NR8R9;
Ra is C1-6 alkyl, C1-6 haloalkyl, halo, cyano, or —OR6;
Rb is hydrogen or C1-6 alkyl;
R2 is optionally substituted 5-membered heteroaryl or optionally substituted 5-membered heterocyclyl;
R3 is hydrogen, C1-6 alkyl, or C1-6 haloalkyl;
R4 is C1-6 alkyl, C1-6 haloalkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein the cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one, two, or three independent occurrences of halo, C1-6 alkyl, C1-6 haloalkyl, cyano, or —OR6;
R5 is hydrogen, C1-6 alkyl, C1-6 haloalkyl, cycloalkyl, or aryl, wherein the cycloalkyl or aryl is optionally substituted with one, two, or three independent occurrences of halo, C1-6 alkyl, C1-6 haloalkyl, cyano, or —OR6 or
R4 and R5, taken together with the carbon atom to which they are attached form a ring;
R6 is hydrogen, C1-6 alkyl, or C1-6 haloalkyl;
each R8 and R9 is independently hydrogen, C1-6 alkyl, —C(O)R10, or —C(O)OR10;
R10 is C1-6 alkyl or C1-6 haloalkyl;
Rx is —O or absent, wherein when Rx is —O, the nitrogen atom of the pyridyl ring is positively charged and Rx is negatively charged, thereby forming a pyridyl N-oxide;
m is 0, 1, 2, or, 3; and
n is 0 or 1, wherein if n is 0, then R5 is hydrogen and R4 is absent.
|