| CPC C07D 215/40 (2013.01) [C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 405/12 (2013.01); C07D 409/12 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01); A61K 45/06 (2013.01)] | 15 Claims |
|
1. A compound of Formula I:
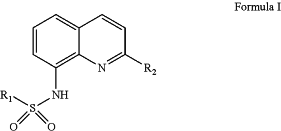 wherein
R1 is selected from the group comprising: saturated or unsaturated C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 cycloalkyl, C3-C6 cycloalkylmethyl, phenyl or benzyl optionally substituted with one or more halogen, C1-C6 alkyl, C1-C6 haloalkyl, OR3, SR3, SO2R3, SO2NR3R4, NR3R4, NR3CO2R4, NO2, CN, CHO, COR3, CO2R3 or CONR3R4, 5- or 6-membered heterocyclyl or heterocyclylmethyl, 5- or 6-membered heteroaryl optionally substituted with one or more halogen, hydroxy, OR3, C1-C6 alkyl or CO2R3, and 5- or 6-membered heteroarylmethyl optionally substituted with one or more halogen or C1-C6 alkyl;
R2 is selected from the group comprising C1-C6 alkyl(heterocyclyl), (C1-C6 alkyl)NR7R8, (C1-C6 alkyl)NR7(C1-C6 alkyl)NR8R9, (C1-C6 alkyl)N((C1-C6 alkyl)NR8R9)2, (C1-C6 alkyl)NR7(C1-C6 alkyl)OR8, (C1-C6 alkyl)NR7(C1-C6 alkyl)O(C1-C6 alkyl)OR8, (C1-C6 alkyl)NR7C(═O)R8, (C1-C6 alkyl)NR7(C1-C6 alkyl)C(═O)NR8R9, (C1-C6alkyl)NR3C(═NNO2)NR4R5, (C1-C6 alkyl)NR3C(═NR4)NR5R6, (C1-C6 alkyl)NR3C(═NR4)R5, (C1-C6 alkyl)NR3SO2R4, (C0-C3 alkyl)CH═NOR7, (C0-C3 alkyl)CH═NNR7R8, (C0-C3 alkyl)CH═NNR3C(═O)R7, (C0-C3 alkyl)CH═NNR3C(═S)R7, (C0-C3 alkyl)CH═NNR3C(═O)NR7R8, (C0-C3 alkyl)CH═NNR3C(═S)NR7R8, (C0-C3 alkyl)C(═O)NR7R8, (C0-C3 alkyl)C(═O)NR3OR7, (C0-C3 alkyl)C(═O)NR3NR7R8;
provided that R1 is not methyl or ethyl when R2 is a methyl(heterocyclyl) group;
R3, R4, R5 and R6 are each selected from the group comprising hydrogen, C1-C6 alkyl, C1-C6 haloalkyl, and aryl; and
R7, R8 and R9 are each selected from the group comprising hydrogen, saturated or unsaturated C1-C6 alkyl, cycloalkyl, aryl, heterocyclyl, heteroaryl, C1-C6 alkyl(cycloalkyl), aralkyl,
C1-C6 alkyl(heterocyclyl), C1-C6 alkyl(heteroaryl), each of which is optionally substituted with one or more of halogen, OR3, NR3R4, NR3COR4, NR3C(═NR4)NR5R6, NR3C(═NNO2)NR4R5, NR3SO2R4, NO2, and CO2R3;
or a pharmaceutically acceptable salt or hydrate thereof.
|