| CPC A61F 2/2418 (2013.01) [A61F 2/2409 (2013.01); A61F 2/2433 (2013.01); A61F 2210/0004 (2013.01); A61F 2210/0057 (2013.01); A61F 2220/0075 (2013.01); A61F 2230/0052 (2013.01); A61F 2230/0054 (2013.01); A61F 2230/0067 (2013.01); A61F 2250/001 (2013.01); A61F 2250/0012 (2013.01); A61F 2250/0018 (2013.01); A61F 2250/0021 (2013.01); A61F 2250/0039 (2013.01); A61F 2250/0048 (2013.01); A61F 2250/0089 (2013.01)] | 19 Claims |
|
1. A hybrid prosthetic heart valve adapted for post-implant expansion and having an inflow end and an outflow end, comprising:
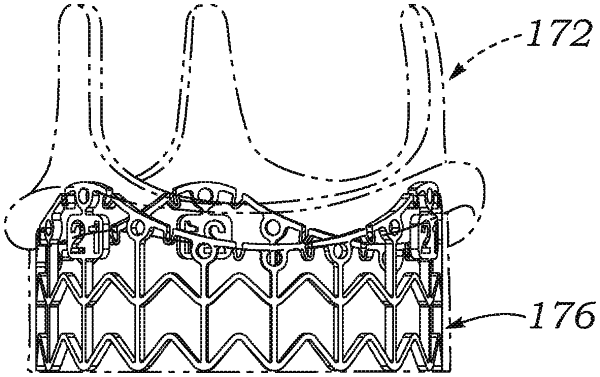
a valve member including an inner structural support stent having upstanding commissure posts extending in the outflow direction alternating with arcuate inflow cusps, and an inflow end undulating up and down corresponding to the commissure posts and cusps, the support stent having a continuous circumferential base defining an implant circumference that is non-compressible in normal physiological use and has a first diameter, and wherein the support stent permits expansion from the first diameter to a second diameter larger than the first diameter upon application of an outward dilatory force from within the support stent substantially larger than forces associated with normal physiological use, and a plurality of flexible leaflets having peripheral edges attached along the commissure posts and inflow cusps of the support stent and configured to ensure one-way blood flow through the valve member; and
a plastically-expandable inflow stent frame secured to and projecting from an inflow end of the support stent and having a strength requiring a predetermined expansion force to convert to an expanded state, wherein the inflow stent frame comprises a plurality of expandable struts, and further wherein an upper edge extends continuously around a periphery of the inflow stent frame and defines an implant circumference with a nominal diameter that enables physiological functioning of the valve member when implanted, and wherein the upper edge is configured to expand only a limited amount from the nominal diameter to an enlarged diameter larger than the nominal diameter upon application of an outward dilatory force from within the outflow end substantially larger than forces associated with normal physiological use, and wherein the upper edge undulates with peaks and valleys to at least partially conform to the inflow end of the support stent.
|