| CPC A61B 6/481 (2013.01) [A61B 5/0066 (2013.01); A61B 5/0075 (2013.01); A61B 5/02007 (2013.01); A61B 5/055 (2013.01); A61B 5/7267 (2013.01); A61B 5/742 (2013.01); A61B 5/7475 (2013.01); A61B 6/032 (2013.01); A61B 6/037 (2013.01); A61B 6/504 (2013.01); A61B 6/5205 (2013.01); A61B 6/5217 (2013.01); A61B 8/12 (2013.01); A61B 8/14 (2013.01); A61K 49/04 (2013.01); G06F 18/10 (2023.01); G06T 7/0012 (2013.01); G06V 10/20 (2022.01); G06V 10/245 (2022.01); G06V 10/761 (2022.01); G06V 10/764 (2022.01); G06V 40/14 (2022.01); G06T 2207/10081 (2013.01); G06T 2207/10088 (2013.01); G06T 2207/10101 (2013.01); G06T 2207/10132 (2013.01); G06T 2207/20081 (2013.01); G06T 2207/30048 (2013.01); G06T 2207/30101 (2013.01); G06V 10/247 (2022.01)] | 30 Claims |
|
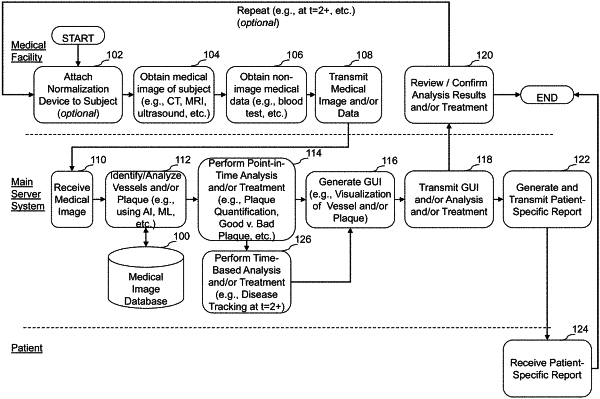
1. A computer-implemented method of determining continued treatment for a subject with atherosclerotic cardiovascular disease (ASCVD) risk based on coronary CT angiography (CCTA) analysis using one or more quantitative imaging algorithms, the method comprising:
assessing, by a computer system, baseline CCTA analysis results of the subject, wherein the baseline CCTA analysis results are generated by applying one or more quantitative imaging algorithms to a first medical image of the subject obtained at a first point in time, the baseline CCTA analysis results comprising one or more atherosclerosis parameters or perivascular tissue parameters, the one or more atherosclerosis parameters comprising one or more of presence, locality, extent, severity, or type of atherosclerosis;
accessing, by the computer system, a second medical image of the subject obtained at a second point in time after applying an initial treatment to the subject, the initial treatment determined based at least in part on the baseline CCTA analysis results of the subject, the initial treatment for the subject comprising one or more of medication, lifestyle, or interventional therapy;
assessing, by the computer system, subject response to the initial treatment by:
applying one or more quantitative imaging algorithms to the second medical image of the subject to generate subsequent CCTA analysis results, the subsequent CCTA analysis results comprising the one or more atherosclerosis parameters or perivascular tissue parameters;
determining one or more changes between the baseline CCTA analysis results and the subsequent CCTA analysis results;
generating an ASCVD risk of the subject at the second point in time based at least in part on the subsequent CCTA analysis results and the determined one or more changes between the baseline CCTA analysis results and the subsequent CCTA analysis results; and
assessing subject response to the initial treatment based at least in part on the generated ASCVD risk of the subject at the second point in time, wherein the subject response is assessed based on one or more of progression, stabilization, or regression of ASCVD risk; and
causing, by the computer system, display of the subject response to the initial treatment, wherein the displayed subject response to the initial treatment is configured to be used to determine a continued proposed treatment for the subject, the continued proposed treatment comprising a higher tiered approach than the initial treatment when the assessed subject response comprises progression of ASCVD, wherein the continued personalized proposed treatment comprises one or more of medication, lifestyle, or interventional therapy,
wherein the computer system comprises a computer processor and an electronic storage medium.
|