| CPC H01M 4/366 (2013.01) [C01B 32/194 (2017.08); C01B 32/198 (2017.08); H01M 4/387 (2013.01); H01M 4/485 (2013.01); H01M 4/587 (2013.01); C01P 2002/72 (2013.01); C01P 2004/03 (2013.01); C01P 2004/04 (2013.01); C01P 2004/80 (2013.01); C01P 2006/40 (2013.01)] | 19 Claims |
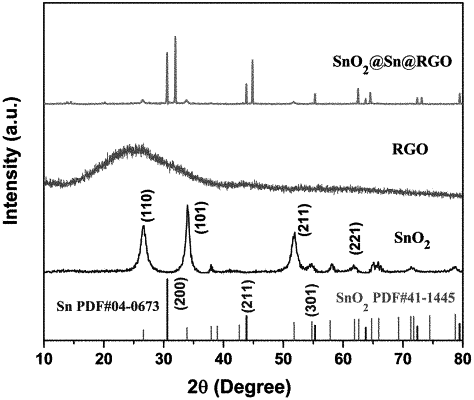
|
1. A preparation method of SnO2@Sn coated RGO (reduced graphene oxide) composite material, comprising:
step 1: weighing a stannate and an organic compound, and dissolving the stannate and the organic compound in deionized water and organic solvent to obtain a solution with a concentration of 1.0 mol/L, and stirring the solution for 0.5 hours to obtain a milky white solution;
step 2: transferring the milky white solution obtained in step 1 into a polytetrafluoroethylene-lined high-pressure hydrothermal reactor, and holding at 150-220° C. for 15-30 hours;
step 3: cooling the solution in step 2, repeatedly centrifuging the cooled solution with deionized water and anhydrous ethanol at a centrifugal rate of 5000-10000 r/m; removing solution to obtain a white precipitate;
step 4: drying the white precipitate obtained in step 3 at 60-120° C. for 12-24 hours to obtain a white powder;
step 5: slowly adding a nitrate to a solution containing a strong acid, and stirring in ice bath for 0.5 hours;
step 6: cooling the solution in step 5 to −10-5° C., slowly adding graphite powder and strong oxidant, stirring in ice bath for 1-5 hours, and after cooling to room temperature, stirring at room temperature for 1-12 hours;
step 7: adding deionized water to the solution obtained in step 6, holding a temperature at 90-100° C., stirring for 0.5 hours, such that a color of the solution obtained in step 6 changes from dark green to bright yellow, and then cooling to room temperature:
step 8: adding an inorganic compound solution with reducibility to the bright yellow solution in step 7, stirring for 1 hour, standing for 10-24 hours, and pouring off a supernatant for 1-5 times;
step 9: adding deionized water to the solution obtained in step 8, and then stirring for 0.5-3 hours, and pouring off a supernatant to obtain a dark yellow solution;
step 10: slowly adding a strong base to the dark yellow solution obtained in step 9 until the dark yellow solution is neutral to obtain a brown solution;
step 11: adding deionized water to the brown solution obtained in step 10, and then stirring for 1-5 hours, and pouring off a supernatant;
step 12: adding an inorganic strong acid to the solution obtained in step 11, washing one time, and stirring for 0.5 hours;
step 13: cooling the solution obtained in step 12, and then centrifuging repeatedly for 0.1-1 hours with deionized water and anhydrous ethanol in a mass ratio of 1:2-8 at a centrifugal rate of 5000-10000 r/m, and pouring off supernatant repeatedly to obtain a black precipitate;
step 14: freeze-drying the black precipitate obtained in step 13 for 12-36 hours to obtain a black powder;
step 15: weighing the white powder obtained in step 4 and the black powder obtained in step 14 with a mass ratio of 3:0.1-10, and dissolving the weighed powder in deionized water, and ultrasonically dispersing for 0.5 hours;
step 16: drying the solution obtained in step 15 at 50-120° C. for 12-36 hours to obtain a black colloid;
step 17: heating the black colloid obtained in step 16 in an inert atmosphere from a temperature of 25° C. to 600-950° C. at a heating rate of 1-5° C./min; and then holding for 2-5 hours, and cooling to room temperature naturally to obtain the SnO2@Sn coated reduced graphene oxide composite material (SnO2@Sn@RGO).
|