| CPC C07D 405/12 (2013.01) [C07C 311/49 (2013.01); C07D 205/04 (2013.01); C07D 207/08 (2013.01); C07D 211/24 (2013.01); C07D 213/54 (2013.01); C07D 223/04 (2013.01); C07D 231/12 (2013.01); C07D 233/64 (2013.01); C07D 241/04 (2013.01); C07D 257/04 (2013.01); C07D 265/30 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01)] | 8 Claims |
|
1. A compound of formula I′:
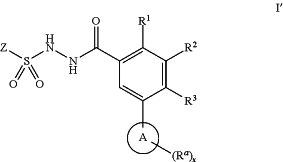 or a pharmaceutically acceptable salt thereof, wherein:
Z is selected from
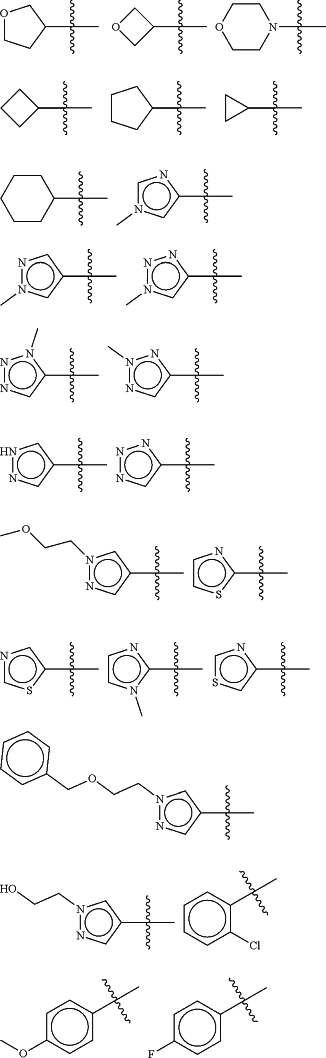   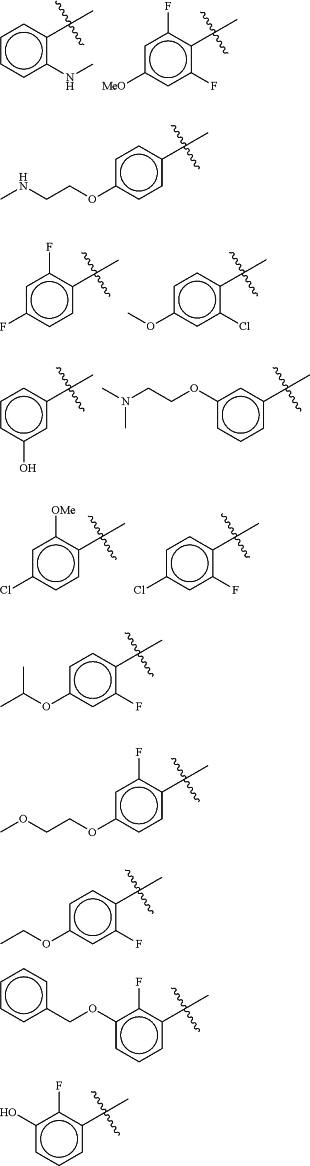 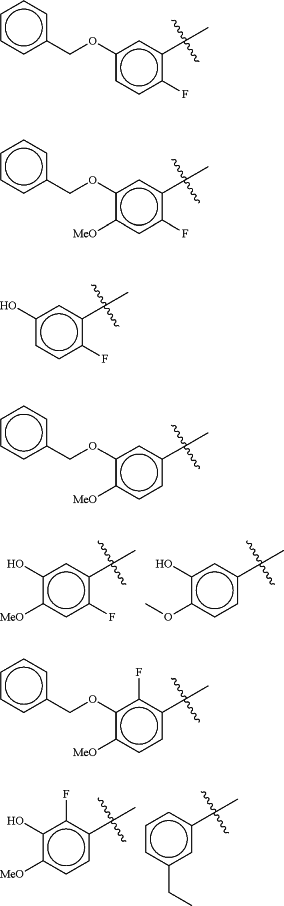 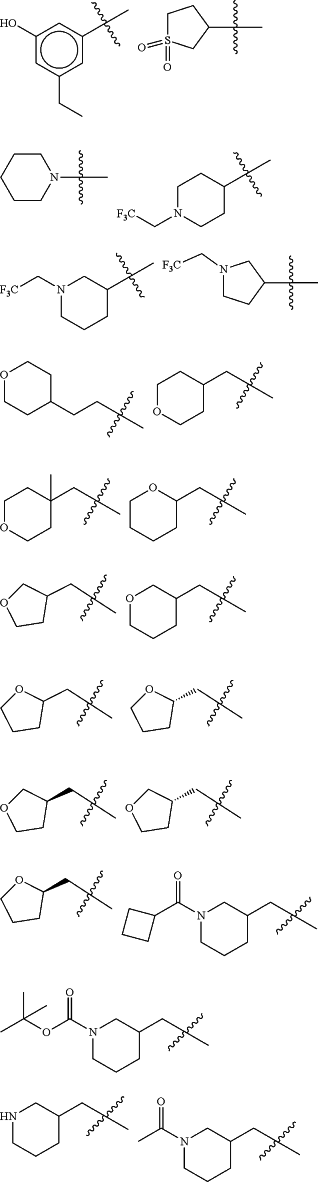 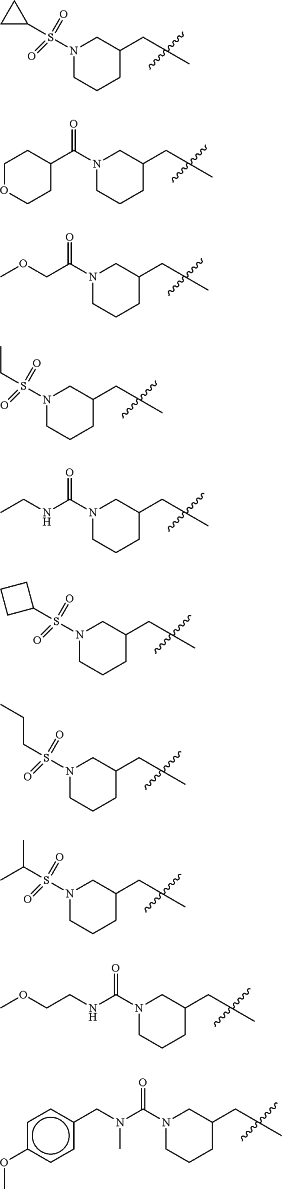 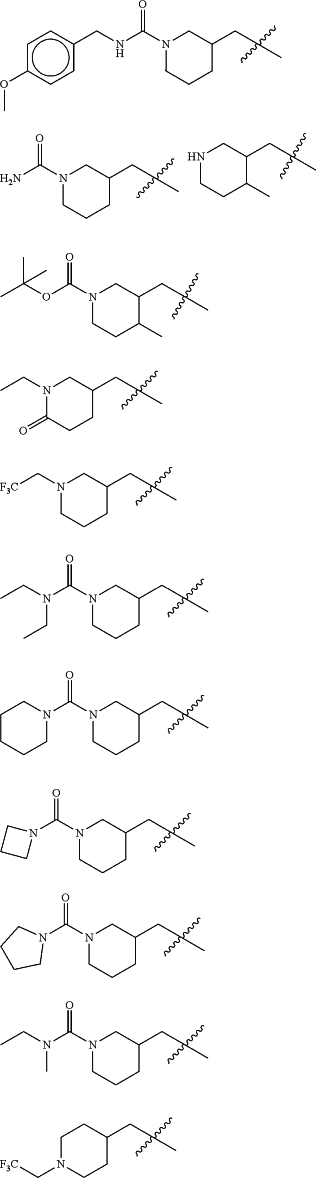 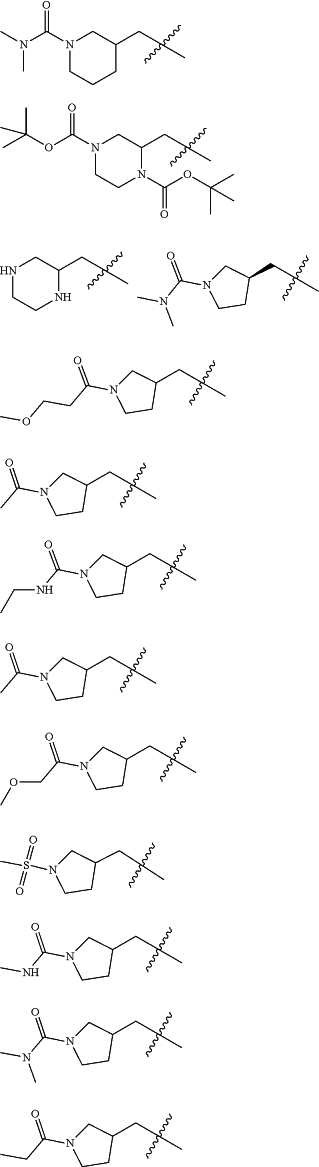 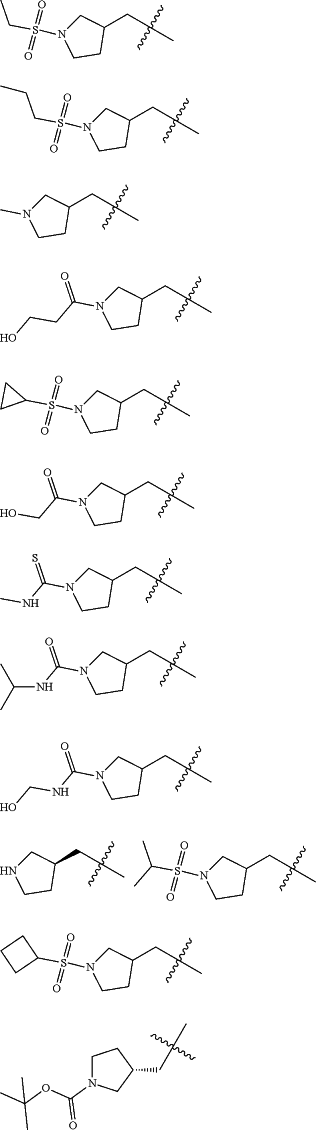 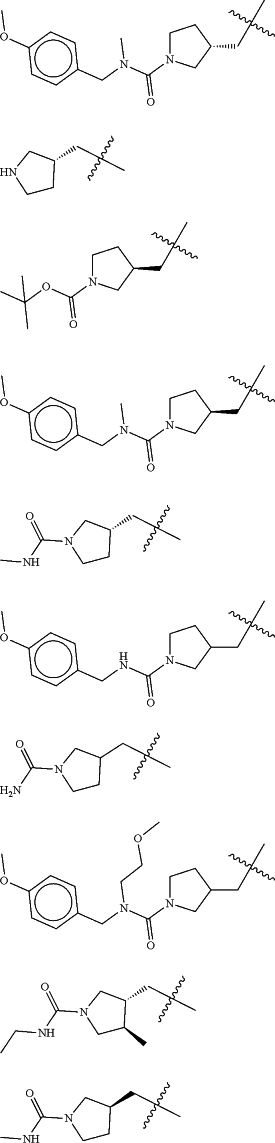 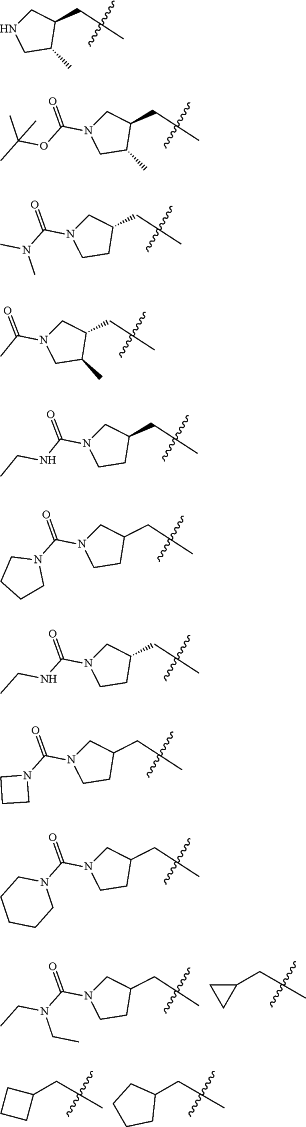 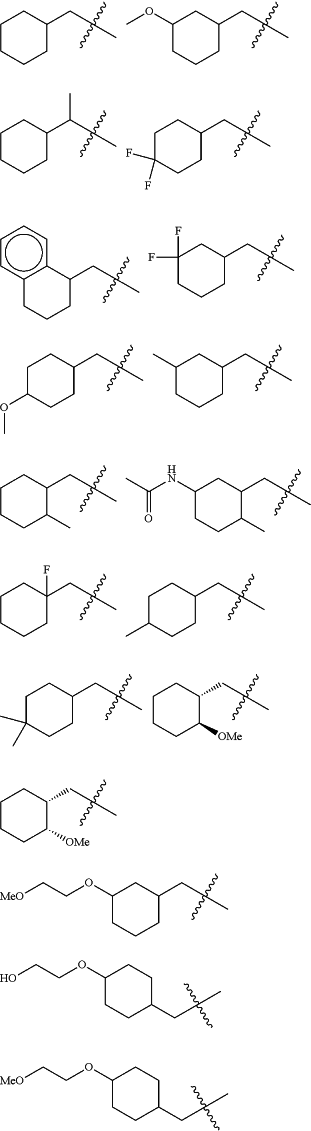 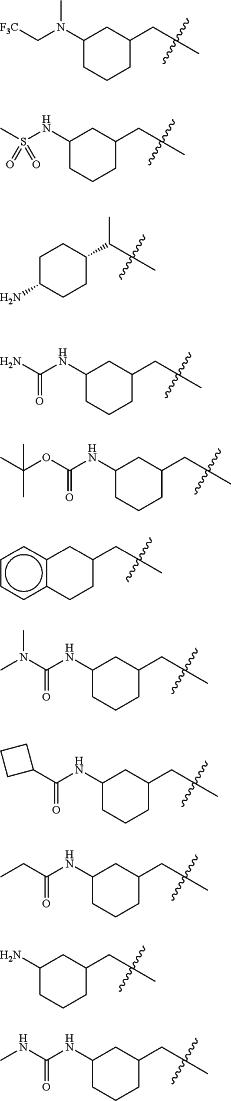 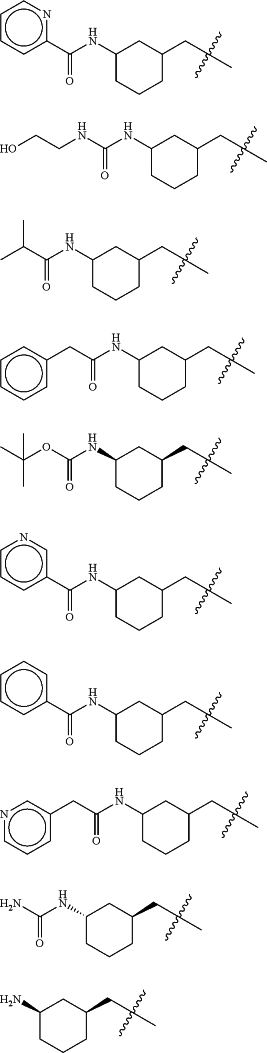 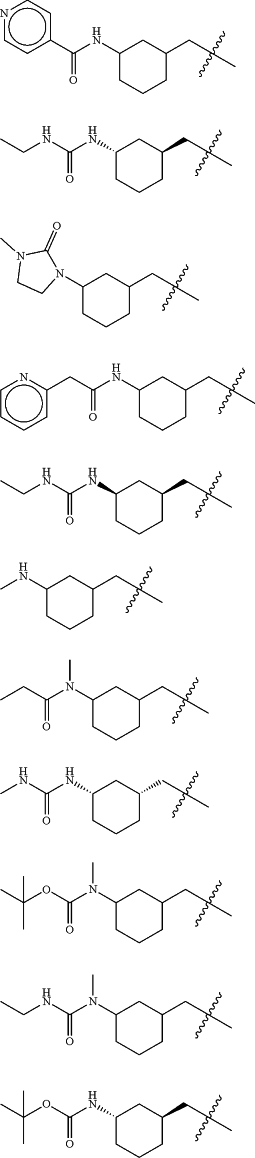 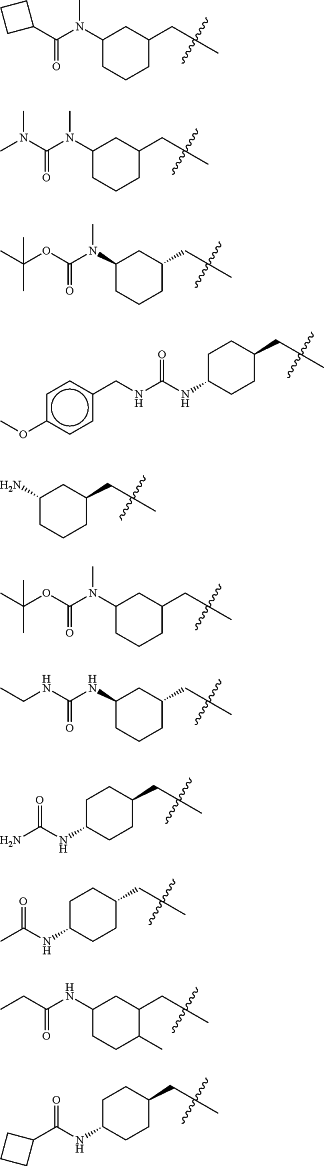 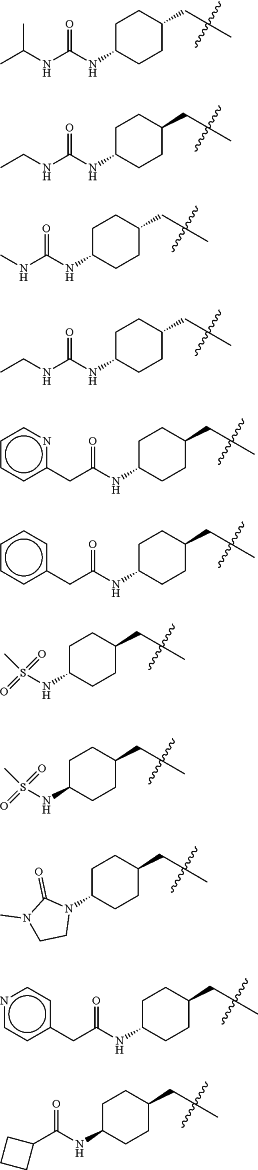 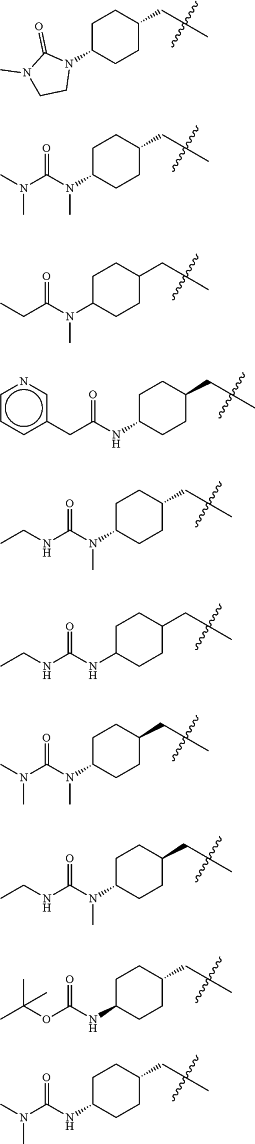 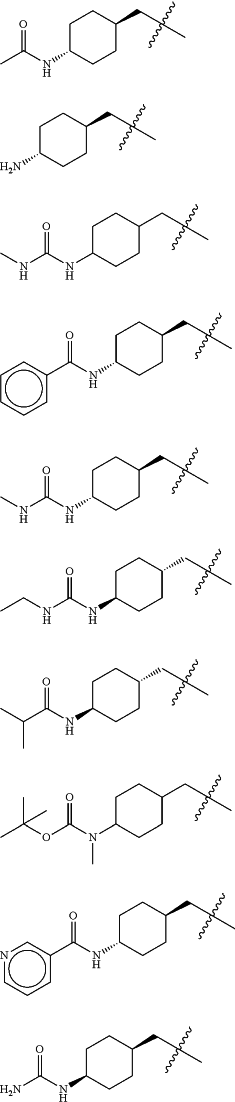 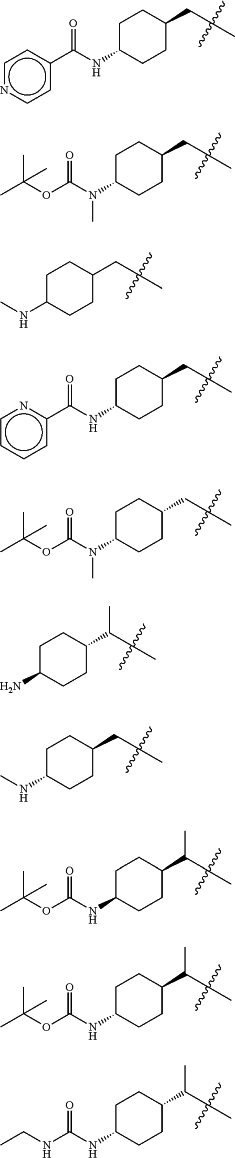 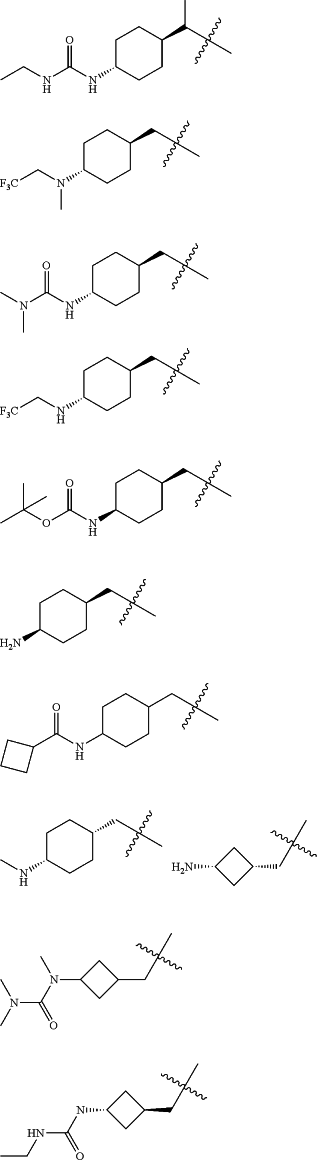 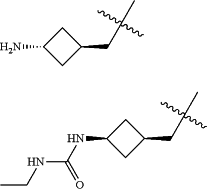 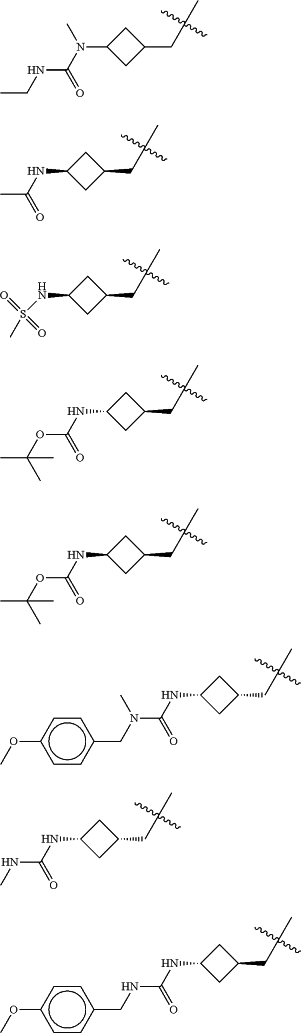 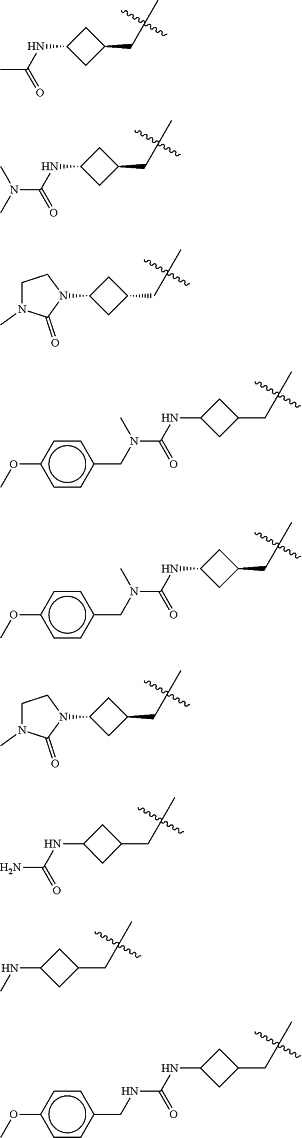 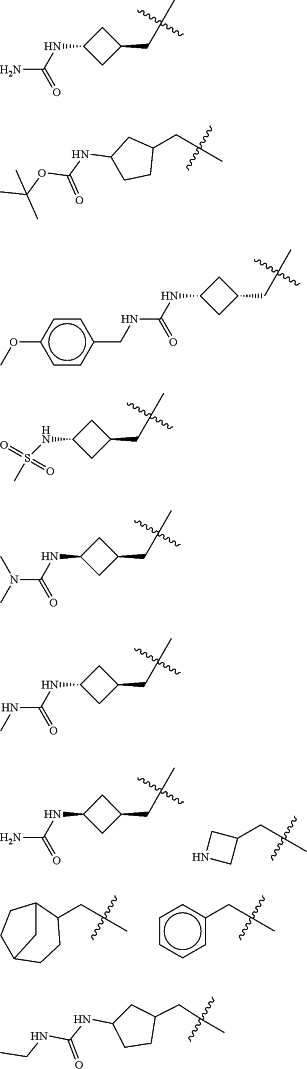 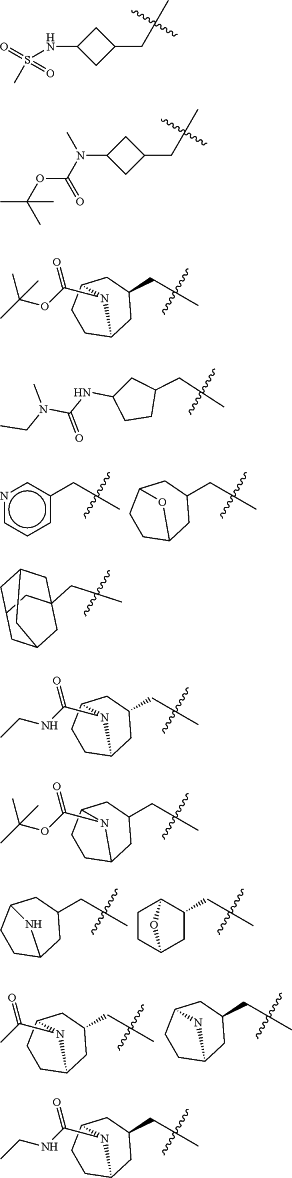 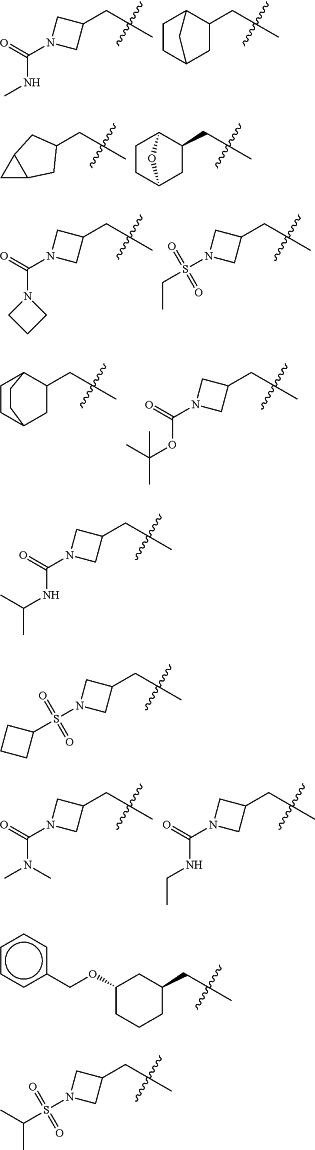 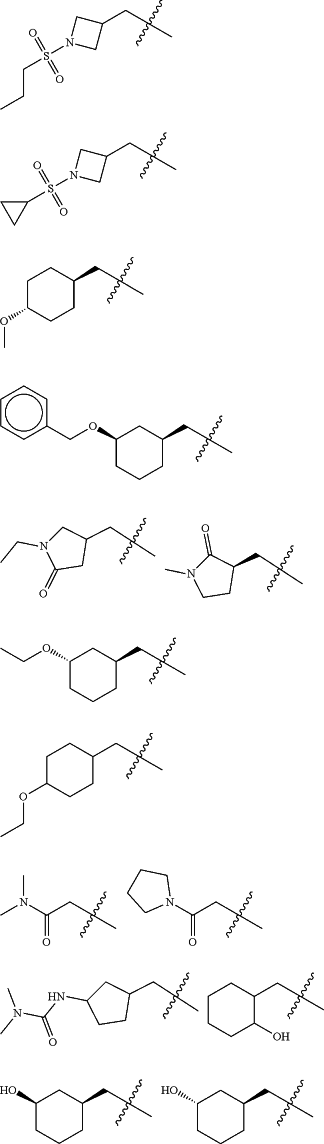 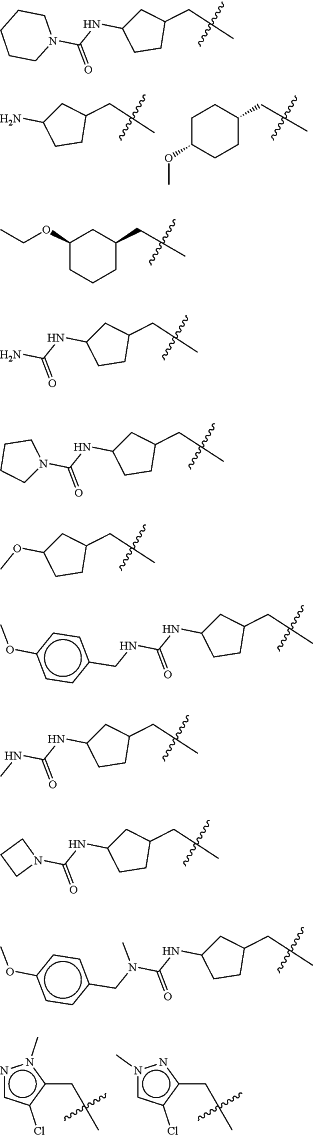 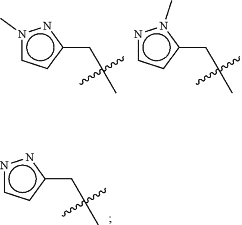 each of R1 and R2 is independently selected from halogen and C1-4 aliphatic;
R3 is selected from hydrogen, halogen, —CN, —NR2, and optionally substituted C1-4 aliphatic;
each R is independently selected from hydrogen, optionally substituted C1-4 aliphatic, and —C(O)O(C1-4 aliphatic);
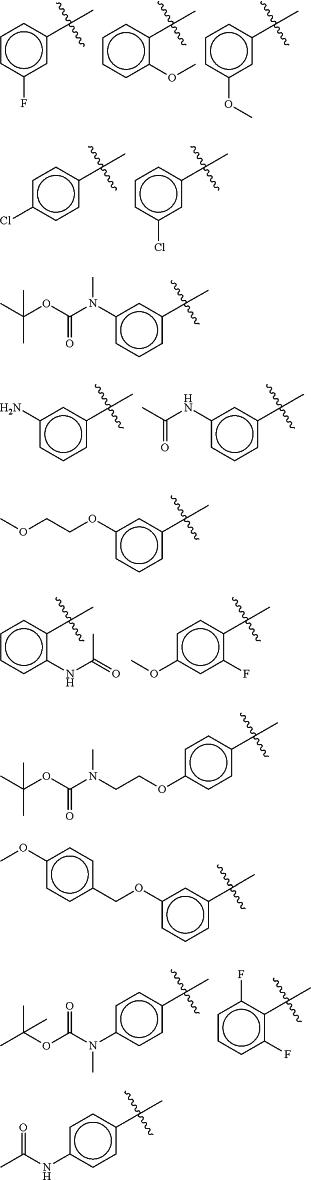
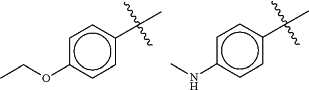
Ring A is an optionally substituted 5- or 6-membered heteroaryl ring having 1-4 heteroatoms independently selected from nitrogen, oxygen and sulfur;
each Ra is selected from halogen and optionally substituted C1-4 aliphatic; and
x is 0-3;
wherein optional substituents are independently selected from monovalent and divalent substituents,
each optional monovalent substituent on a substitutable carbon atom is independently selected from halogen; —(CH2)0-4R∘; —(CH2)0-4OR∘; —O(CH2)0-4R∘, —O—(CH2)0-4C(O)OR∘; —(CH2)0-4CH(OR∘)2; —(CH2)0-4SR∘; —(CH2)0-4Ph, optionally substituted with R∘; —(CH2)0-4O(CH2)0-1Ph optionally substituted with R∘; —CH═CHPh optionally substituted with R∘; —(CH2)0-4O(CH2)0-1-pyridyl optionally substituted with R∘; —NO2; —CN; —N3; —(CH2)0-4N(R∘)2; —(CH2)0-4N(R∘)C(O)R∘; —N(R∘)C(S)R∘; —(CH2)0-4N(R∘)C(O)NR∘2; —N(R∘)C(S)NR∘2; —(CH2)0-4N(R∘)C(O)OR∘; —N(R∘)N(R∘)C(O)R∘; —N(R∘)N(R∘)C(O)NR∘2; —N(R∘)N(R∘)C(O)OR∘; —(CH2)0-4C(O)R∘; —C(S)R∘; —(CH2)0-4C(O)OR∘; —(CH2)0-4C(O)SR∘; —(CH2)0-4C(O)OSiR∘3; —(CH2)0-4OC(O)R∘; —OC(O)(CH2)0-4SR∘; —(CH2)0-4SC(O)R∘; —(CH2)0-4C(O)NR02; —C(S)NR∘2; —C(S)SR∘; —SC(S)SR∘, —(CH2)0-4OC(O)NR∘2; —C(O)N(OR∘)R∘; —C(O)C(O)R∘; —C(O)CH2C(O)R∘; —C(NOR∘)R∘; —(CH2)0-4SSR∘; —(CH2)0-4S(O)2R∘; —(CH2)0-4S(O)2OR∘; —(CH2)0-40S(O)2R∘; —S(O)2NR∘2; —(CH2)0-4S(O)R∘; —N(R∘)S(O)2NR∘2; —N(R∘)S(O)2R∘; —N(OR∘)R∘; —C(NH)NR∘2; —P(O)2R∘; —P(O)R∘2; —OP(O)R∘2; —OP(O)(OR∘)2; SiR∘3; —(C1-4 straight or branched alkylene)O—N(R∘)2; or —(C1-4 straight or branched alkylene)C(O)O—N(R∘)2;
wherein each R∘ is optionally substituted and is independently hydrogen, C1-6 aliphatic, —CH2Ph, —O(CH2)0-1Ph, —CH2-(5-6 membered heteroaryl ring), or a 3-6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
or two independent occurrences of R∘ are taken together with their intervening atom(s) form an optionally substituted 3- to 12-membered saturated, partially unsaturated, or aryl mono- or bicyclic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
wherein each optional monovalent substituent bound to R∘ is independently halogen, —(CH2)0-2R●, -(haloR●), —(CH2)0-2OH, —(CH2)0-2OR●, —(CH2)0-2CH(OR●)2, —O(haloR●), —CN, —N3, —(CH2)0-2C(O)R●, —(CH2)0-2C(O)OH, —(CH2)0-2C(O)OR●, —(CH2)0-2SR●, —(CH2)0-2SH, —(CH2)0-2NH2, —(CH2)0-2NHR●, —(CH2)0-2NR●2, —NO2, —SiR●3, —OSiR●3, —C(O)SR●, —(C1-4 straight or branched alkylene)C(O)OR●, —SSR●, or -Ph optionally substituted with R●;
wherein each R● is unsubstituted or where preceded by “halo” is substituted only with one or more halogens, and is independently C1-4 aliphatic, —CH2Ph, —O(CH2)0-1Ph, or a 5- to 6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur, ═O, or ═S;
wherein each optional divalent substituent on a saturated carbon atom is ═O, ═S, ═NNR*2, ═NNHC(O)R*, ═NNHC(O)OR*, ═NNHS(O)2R*, ═NR*, ═NOR*, —O(C(R*2))2-3O—, or —S(C(R*2))2-3S—, or wherein a divalent substituent —O(CR*2)2-3O— is bound to vicinal substitutable carbons;
wherein each occurrence of R* is independently selected from hydrogen, optionally substituted C1-6 aliphatic, or an unsubstituted 5- to 6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
wherein each occurrence of R* is optionally substituted with halogen, —R●, -(haloR●), —OH, —OR●, —O(haloR●), —CN, —C(O)OH, —C(O)OR●, —NH2, —NHR●, —NR●2, or —NO2;
wherein each R● is unsubstituted or where preceded by “halo” is substituted only with one or more halogens, and is independently C1-4 aliphatic, —CH2Ph, —O(CH2)0-1Ph, or a 5- to 6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
wherein each optional substituent on a substitutable nitrogen atom is independently —R†, —NR†2,
—C(O)R†, —C(O)OR†, —C(O)NR†2, —C(O)C(O)R†, —C(O)CH2C(O)R†, —S(O)2R†, —S(O)2NR†2, —C(S)NR†2, —C(NH)NR†2, or —N(R†)S(O)2R†;
wherein each R† is independently hydrogen, optionally substituted C1-6 aliphatic, unsubstituted —OPh, or an unsubstituted 5- to 6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur, or,
two independent occurrences of R† are taken together with their intervening atom(s) to form an unsubstituted 3- to 12-membered saturated, partially unsaturated, or aryl mono- or bicyclic ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur;
wherein each optional substituent on R† is independently halogen, —R●, -(haloR●), —OH, —OR●, —O(haloR●), —CN, —C(O)OH, —C(O)OR●, —NH2, —NHR●, —NR●, or —NO2,
wherein each R● is unsubstituted or where preceded by “halo” is substituted only with one or more halogens, and is independently C1-4 aliphatic, —CH2Ph, —O(CH2)0-1Ph, or a 5- to 6-membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or sulfur.
|