| CPC C07D 401/14 (2013.01) [A61K 45/06 (2013.01); A61P 31/18 (2018.01); C07B 2200/13 (2013.01)] | 69 Claims |
|
1. A method of preventing an HIV infection in a subject, comprising administering to the subject a compound of Formula (Ia) or Formula (Ib):
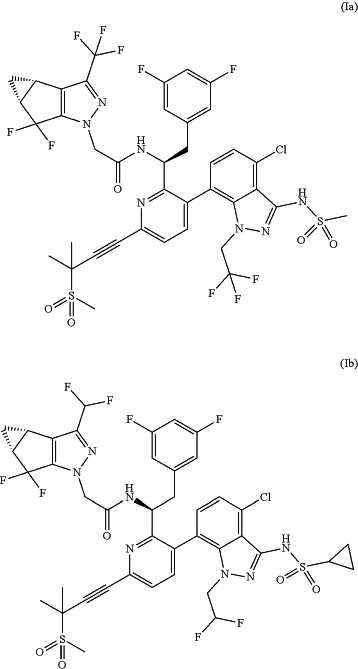 or a pharmaceutically acceptable salt thereof, wherein:
the compound of Formula (Ia) or Formula (Ib), or a pharmaceutically acceptable salt thereof, is administered subcutaneously at a concentration of about 309 mg/mL; or
the compound of Formula (Ia) or Formula (Ib), or a pharmaceutically acceptable salt thereof, is administered intramuscularly at a concentration of about 400 mg/mL to about 500 mg/mL; or
the compound of Formula (Ia) or Formula (Ib), or a pharmaceutically acceptable salt thereof, is administered subcutaneously at a concentration of about 400 mg/mL; or
the compound of Formula (Ia) or Formula (Ib), or a pharmaceutically acceptable salt thereof, is administered subcutaneously at a concentration of about 500 mg/mL.
|