| CPC C07D 239/48 (2013.01) [A61P 35/00 (2018.01); C07D 401/12 (2013.01)] | 14 Claims |
|
1. A compound having the structure of Formula (I):
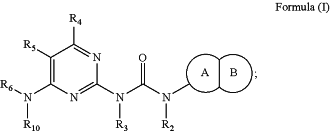 wherein:
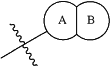 is
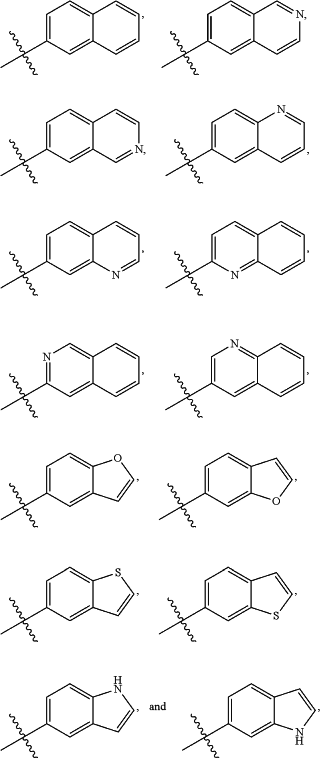 wherein
 is unsubstituted or substituted by 1, 2 or 3 R1 groups;
each R1 is independently halogen, —CN, —NO2, —OH, —OCF3, —OCH2F, —OCF2H, —CF3, —SR8, —N(R8)S(═O)2R9, —S(═O)2N(R8)2, —S(═O)2R9, —S(═O)2R9, —C(═O)R9, —CO2R8, —N(R8)2, —C(═O)N(R8)2, or —N(R8)C(═O)R9;
R2 and R3 are each independently H, or C1-C4alkyl;
R4 and R5 are independently H, halogen, —CN, —OH, —CF3, or;
R6 is H, unsubstituted C1-C6 haloalkyl,
(C(R14)(R15))mN(R11)(R12), —(C(R14)(R15))mOR13, or —OR22;
each R8 is independently H or C1-C6alkyl;
each R9 is independently C1-C6alkyl;
R10 is H or C1-C4alkyl;
R11 is H, or C1-C6alkyl;
R12 is H or C1-C6 alkyl;
R13 is H or C1-C6alkyl;
each R14 and R15 is each independently H, halogen, or C1-C6alkyl;
R22 is H, or C1-C6alkyl;
m is 2-6; and
n is 1-5; or
a pharmaceutically acceptable salt thereof.
|