| CPC A61K 31/437 (2013.01) [A61P 5/44 (2018.01)] | 20 Claims |
|
1. A method of treating a patient suffering from a symptom of hypercortisolemia, or comorbidity thereof, the method comprising administering to the subject an amount of relacorilant, CORT122928, CORT113176, CORT125281, or CORT125329, wherein said amount is selected from 50 milligrams (mg), 100 mg, 150 mg, 200 mg, 250 mg, 300 mg, 350 mg, 400 mg, and 500 mg, effective to treat said symptom or comorbidity of hypercortisolemia,
Wherein relacorilant is:
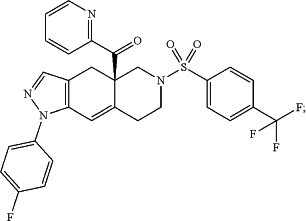
(R)-(1-(4-fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone, which has the formula
 wherein CORT122928 is:
(R)-(1-(4-flurophenyl)-6-((4-(trifluoromethyl)phenyl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(thiazol-2-yl)methanone, termed, having the formula
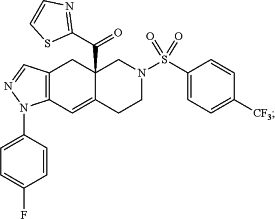 Wherein CORT113176 is:
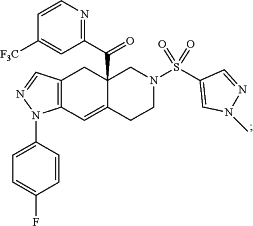
(R)-(1-(4-fluorophenyl)-6-((4-(trifluoromethyl)phenyl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(pyridin-2-yl)methanone, having the formula
 Wherein CORT125281 is:
((4aR,8aS)-1-(4-fluorophenyl)-6-((2-methyl-2H-1,2,3-triazol-4-yl)sulfonyl)-4,4a,5,6,7,8,8a,9-octahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone which has the structure:
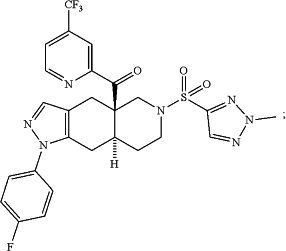 and
Wherein CORT125329 is:
((4aR,8aS)-1-(4-fluorophenyl)-6-((2-isopropyl-2H-1,2,3-triazol-4-yl)sulfonyl)-4,4a,5,6,7,8,8a,9-octahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(thiazol-2-yl)methanone, having the formula:
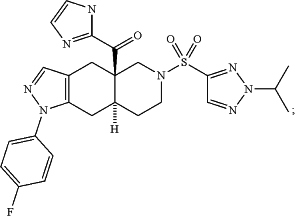 and
wherein said symptom or comorbidity of hypercortisolemia is one or more of:
hyperglycemia, wherein said treatment is effective to lower the patient's AUCglucose by at least about 15% as compared to the patient's baseline AUCglucose measured prior to treatment;
hypertension, wherein said treatment is effective to lower the patient's 24-hour mean systolic or 24 hour mean diastolic blood pressure by at least about 5 millimeters of mercury (mmHg) as compared to the patient's baseline blood pressure measured prior to treatment;
an abnormal liver enzyme level, wherein said treatment is effective to lower an abnormal patient liver enzyme level as compared to the patient's baseline liver enzyme level measured prior to treatment;
an abnormal fructosamine level, wherein said treatment is effective to improve an abnormal patient fructosamine level as compared to the patient's baseline fructosamine level measured prior to treatment;
an abnormal serum osteocalcin level, wherein said treatment is effective to improve an abnormal patient serum osteocalcin level by at least 1 microgram per liter as compared to the patient's baseline serum osteocalcin level measured prior to treatment;
an abnormal heartbeat interval or median heart rate, wherein said treatment is effective to improve an abnormal patient heartbeat interval or median heart rate as compared to the patient's baseline heartbeat interval or median heart rate measured prior to treatment;
an abnormal blood coagulation measure, wherein said treatment is effective to improve an abnormal patient blood coagulation measure as compared to the patient's baseline blood coagulation as measured prior to treatment;
an abnormal blood cell measure, wherein said treatment is effective to improve an abnormal patient blood cell measure as compared to the patient's baseline blood cell measure as measured prior to treatment;
an abnormal adrenocorticotropic hormone (ACTH) or pro-opiomelanocortin (POMC) level, wherein said treatment is effective to improve an abnormal patient ACTH or POMC level as compared to the patient's baseline ACTH or POMC level as measured prior to treatment;
improve quality of life in a Cushing's patient, wherein said treatment is effective to improve patient quality of life as measured by Cushing Quality of Life Score, as compared to the patient's baseline quality of life as so measured prior to treatment;
improve cognition in a Cushing's patient, wherein said treatment is effective to improve patient cognition as measured by a Trail Making cognitive test, as compared to the patient's baseline cognition as measured by said cognitive test prior to treatment;
lessen patient depression, as measured by the Beck Depression Scale, wherein said treatment is effective to reduce patient depression as indicated by the Beck Depression Scale as compared to the patient's baseline depression as measured prior to treatment;
whereby the patient suffering from hypercortisolemia and a symptom or comorbidity thereof is treated and the symptom or comorbidity is improved.
|