| CPC A61B 18/1492 (2013.01) [A61B 1/00082 (2013.01); A61B 1/00096 (2013.01); A61B 1/05 (2013.01); A61B 1/0676 (2013.01); A61B 1/313 (2013.01); A61B 8/12 (2013.01); A61B 8/445 (2013.01); A61B 8/4494 (2013.01); A61M 25/10 (2013.01); A61M 25/1011 (2013.01); A61B 5/01 (2013.01); A61B 5/0538 (2013.01); A61B 5/6853 (2013.01); A61B 5/6858 (2013.01); A61B 18/1206 (2013.01); A61B 18/1815 (2013.01); A61B 90/30 (2016.02); A61B 90/361 (2016.02); A61B 90/37 (2016.02); A61B 2017/003 (2013.01); A61B 2017/0011 (2013.01); A61B 2017/00106 (2013.01); A61B 2017/00867 (2013.01); A61B 2017/22038 (2013.01); A61B 2018/00011 (2013.01); A61B 2018/0016 (2013.01); A61B 2018/0022 (2013.01); A61B 2018/00023 (2013.01); A61B 2018/00029 (2013.01); A61B 2018/00083 (2013.01); A61B 2018/0088 (2013.01); A61B 2018/00214 (2013.01); A61B 2018/00232 (2013.01); A61B 2018/00238 (2013.01); A61B 2018/00267 (2013.01); A61B 2018/00285 (2013.01); A61B 2018/00291 (2013.01); A61B 2018/00351 (2013.01); A61B 2018/00357 (2013.01); A61B 2018/00375 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00613 (2013.01); A61B 2018/00642 (2013.01); A61B 2018/00702 (2013.01); A61B 2018/00744 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00797 (2013.01); A61B 2018/00815 (2013.01); A61B 2018/00839 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/00898 (2013.01); A61B 2018/00982 (2013.01); A61B 2018/124 (2013.01); A61B 2018/1465 (2013.01); A61B 2090/0454 (2016.02); A61B 2090/064 (2016.02); A61B 2090/065 (2016.02); A61B 2090/309 (2016.02); A61B 2090/364 (2016.02); A61B 2090/3614 (2016.02); A61B 2090/371 (2016.02); A61B 2090/373 (2016.02); A61B 2090/3784 (2016.02); A61B 2090/3937 (2016.02); A61B 2090/3966 (2016.02); A61B 2217/007 (2013.01); A61B 2218/002 (2013.01); A61B 2562/125 (2013.01); A61B 2562/164 (2013.01); A61M 25/0108 (2013.01); A61M 25/0133 (2013.01); A61M 25/0147 (2013.01); A61M 25/04 (2013.01); A61M 2025/0681 (2013.01); A61N 1/05 (2013.01); C08L 2201/12 (2013.01)] | 14 Claims |
|
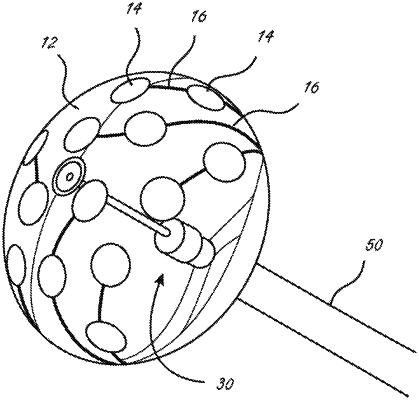
1. An ablation catheter, comprising:
an elongate shaft;
an inflatable balloon positioned at a distal region of the elongate shaft;
a first ablation electrode disposed outside of and carried by an outer surface of the inflatable balloon;
a first ultrasound transducer disposed outside of the inflatable balloon and configured to monitor a characteristic associated with an ablation process; and
a flexible circuit, including a substrate layer, a first conductor and a second conductor, the flexible circuit disposed outside of and carried by the outer surface of the inflatable balloon, the first conductor in electrical communication with the first ablation electrode, and the second conductor in electrical communication with the first ultrasound transducer,
wherein the first ablation electrode is disposed over the substrate layer, and wherein the first ultrasound transducer is disposed on the substrate layer and is encapsulated by the first ablation electrode.
|