| CPC G16H 50/30 (2018.01) [G01N 33/57415 (2013.01); G16H 50/20 (2018.01); G16H 80/00 (2018.01)] | 22 Claims |
|
1. A method of treating breast cancer in an individual in need thereof, the method comprising:
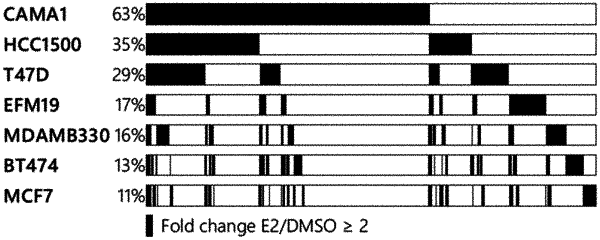
(i) determining an estrogen receptor (ER) pathway activity score from a sample from the individual, wherein the ER pathway activity score from the sample is at or above a reference ER pathway activity score;
wherein the ER pathway activity score is calculated by subtracting an E2-repressed score, determined from the average z-scored expression of at least 4 of the E2-repressed genes selected from the group consisting of BAMBI, BCAS1, CCNG2, DDIT4, EGLN3, FAM171B, GRM4, IL1R1, LIPH, NBEA, PNPLA7, PSCA, SEMA3E, SSPO, STON1, TGFB3, TP53INP1, and TP53INP2, from an E2-induced score, determined from the average z-scored expression of 23 of the E2-induced genes selected from the group consisting of AGR3, AMZ1, AREG, C5AR2, CELSR2, CT62, FKBP4, FMN1, GREB1, IGFBP4, NOS1AP, NXPH3, OLFM1, PGR, PPM1J, RAPGEFL1, RBM24, RERG, RET, SGK3, SLC9A3R1, TFF1, and ZNF703; and
(ii) administering to the individual an effective amount of an endocrine therapy selected from the group consisting of:
(a) a selective estrogen receptor degrader, a selective estrogen receptor modulator, a selective estrogen receptor covalent antagonist, a selective human estrogen receptor agonist, an aromatase inhibitor, or a combination of two or more thereof;
(b) letrozole, anastrozole, exemestane, testolactone, hydroxytamoxifen, clomifene, toremifene, raloxifene, anordrin, bazedoxifene, broparestrol, cyclofenil, lasofoxifene, ormeloxifene, acolbifene, elacestrant, brilanestrant, clomifenoxide, droloxifene, etacstil, ospemifene, G1T48, AZ9496, GDC-0927, LX-039, fulvestrant, or GDC-9545, a pharmaceutically acceptable salt of one of the foregoing, or a combination of two or more of the foregoing; and
(c) a compound having formula:
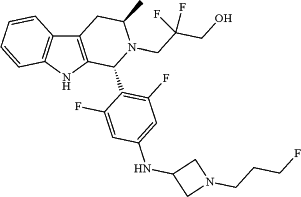 or a pharmaceutically acceptable salt thereof.
|