| CPC C12P 17/10 (2013.01) [C07C 17/278 (2013.01); C07D 209/80 (2013.01); C07D 209/88 (2013.01); C07D 209/94 (2013.01); C07D 295/155 (2013.01); C07B 2200/13 (2013.01)] | 15 Claims |
|
1. A process for preparing an L-arginine salt of (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid of Formula (Ia):
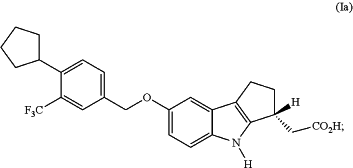 comprising the following steps:
a) hydrolyzing a compound of Formula (IIk):
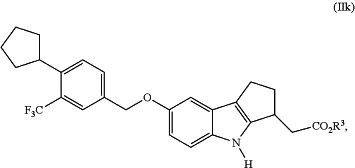 wherein R3 is C1-C6 alkyl;
in the presence of a lipase and a hydrolyzing-step solvent to form said (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid of Formula (Ia); and
b) contacting said (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (Formula (Ia)) with L-arginine or a salt thereof, in the presence of a contacting-step solvent and H2O to form said L-arginine salt of (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid of Formula (Ia),
wherein the molar ratio between (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (Formula (Ia)) and L-arginine is about 1.0: 1.0 to about 1.0: 1.2,
and wherein the contacting in step b) further comprises the step of adding an aqueous solution of L-arginine to a first contacting mixture comprising (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (Formula (Ia)) and the C1-C6 alcohol to form a second contacting mixture,
wherein the first contacting mixture is at a temperature of about 45° C. to about 75° C.
|