| CPC C07H 7/04 (2013.01) [C07H 5/06 (2013.01); C07H 17/02 (2013.01)] | 4 Claims |
|
1. A process for preparing a compound of formula A-5,
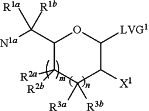 A-5, or a salt thereof,
wherein:
R1a and R1b are independently selected from the group consisting of H, C1-C12 alkyl, C1-C6 cycloalkyl, aryl, and heteroaryl, wherein the alkyl, cycloalkyl, aryl, or heteroaryl is unsubstituted or substituted with one or more substituents independently selected from the group consisting of halogen, cyano, alkyl, aryl, heteroaryl, —SR12, —SO2R13, —OSF2NR14R15, —NR14R15, —N3, and —OR16, and
wherein each R12, R13, R14, R15, and R16 is independently H or alkyl; or
R1a and R1b, together with the atom to which they are attached, form a cycloalkyl group or a heterocycloalkyl group, wherein the cycloalkyl group or heterocycloalkyl group is unsubstituted or substituted with one or more substituents independently selected from the group consisting of halogen, cyano, alkyl, aryl, heteroaryl, —SR22, —SO2R23, —NR24R25, and —OR26, and
wherein each R22, R23, R24, R25, and R26 is independently H or alkyl, wherein the alkyl is unsubstituted or substituted with one or more substituents independently selected from the group consisting of halogen, cyano, NR14R15, and —OR16;
R2a, R2b, R3a and R3b are independently selected from the group consisting of H, —OR27, —NR28R29, halogen, C1-C4 cycloalkyl, and C1-C6 alkyl, wherein each R27, R28, and R29 is independently H, alkyl, amino protecting group, or hydroxyl protecting group; wherein the C1-C6 alkyl or alkyl is unsubstituted or substituted with one or more substituents selected from the group consisting of aryl, halogen, —OR30, —NR31R32, —SR33, and —SO2R34;
wherein each R30, R31, R32, R33, and R34 is independently H or alkyl substituted with one or more substituents independently selected from the group consisting of halogen, cyano, NR14R15, and —OR16; or
R2a and R2b form an oxo or imino group substituted with C1-C6 alkyl;
R3a and R3b form an oxo or imino group substituted with C1-C6 alkyl;
X1 is —F, —Cl, —Br, or —I;
LVG1 is a leaving group;
N1a is —NHPg1a, —N(Pg1a)2, or N3, wherein each Pg1a is independently an amino protecting group;
m is zero, 1, or 2;
n is zero, 1, or 2; and,
wherein m+n is 1, 2 or 3;
the process comprising:
(a) contacting a compound of formula A-1:
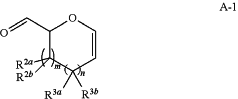 or a salt thereof, with a chiral auxiliary reagent to yield a compound of formula A-2:
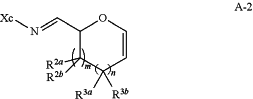 or a salt thereof, wherein Xc is a chiral auxiliary group;
(b) contacting the compound of formula A-2 with a Grignard or organolithium reagent to yield a compound of formula A-3:
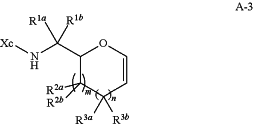 or a salt thereof;
(c) contacting the compound of formula A-3 with a halogen reagent in presence of a nucleophile reagent (Nuc-1) to yield a compound of formula A-4:
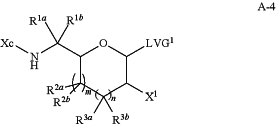 or a salt thereof,
wherein:
X1 is —F, —Cl, —Br, or —I;
Nuc-1 is LVG1-M, wherein M is H, a metal cation, a non-metal cation, or a lone pair of electrons;
LVG1 is a leaving group; and
(d) exchanging the chiral auxiliary group for an amino protecting group in the compound of formula A-4 by reaction with an amino protecting group reagent to yield the compound of formula A-5.
|