| CPC C07D 519/00 (2013.01) [C07D 471/04 (2013.01); C07D 487/04 (2013.01)] | 19 Claims |
|
1. A compound of the Formula II′:
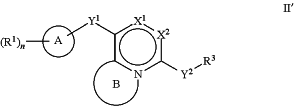 or a pharmaceutically acceptable salt, tautomer, or stereoisomer thereof, wherein:
A is cycloalkyl, heterocyclyl, aryl, or heteroaryl, wherein cycloalkyl, heterocyclyl, aryl, and heteroaryl are 5- to 12-membered monocyclic or 5- to 12-membered polycyclic;
R1 is independently, at each occurrence, —H, —C1-C6alkyl, —C2-C6alkenyl, —C4-C8cycloalkenyl, —C2-C6alkynyl, —OH, —OR6, halogen, —NO2, —CN, —NR5R6, —SR5, —S(O)2NR5R6, —S(O)2R5, —NR5S(O)2NR5R6, —NR5S(O)2R6, —S(O)NR5R6, —S(O)R5, —NR5S(O)NR5R6, —NR5S(O)R6, —C(O)R5, —CO2R5, —C(O)NR5R6, —NR5C(O)R6, monocyclic or polycyclic heterocyclyl, spiroheterocyclyl, or oxo, wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, heterocyclyl, or spiroheterocyclyl is optionally substituted with one or more —OH, halogen, —NO2, oxo, ═O, —CN, —R5, —OR5, —NR5R6, —SR5, —S(O)2NR5R6, —S(O)2R5, —NR5S(O)2NR5R6, —NR5S(O)2R6, —S(O)NR5R6, —S(O)R5, —NR5S(O)NR5R6, —NR5S(O)R6, heterocycle, aryl, or heteroaryl;
Y1 is —S—, a direct bond, —NH—, —S(O)2—, —S(O)2—NH—, —C(═CH2)—, —CH2—, or —S(O)—;
X1 is N or CR2;
X2 is N or CH;
B, including the atoms at the points of attachment, is
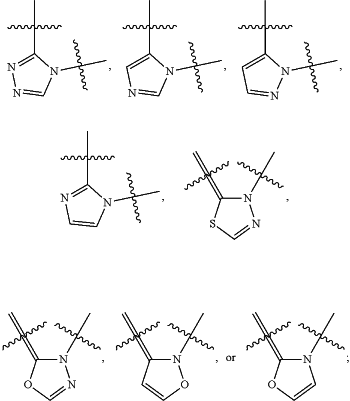 Y2 is —NRa—; wherein the bond on the left side of Y2, as drawn, is bound to the ring and the bond on the right side of the Y2 moiety, as drawn, is bound to R3;
Rb is independently, at each occurrence, —H, —OH, —C1-C6alkyl, —C3-C8cycloalkyl, —C2-C6alkenyl, —(CH2)n-aryl, heterocyclyl containing 1-5 heteroatoms selected from the group consisting of N, S, P, and O, or heteroaryl containing 1-5 heteroatoms selected from the group consisting of N, S, P, and O; wherein each alkyl, cycloalkyl, alkenyl, heterocycle, heteroaryl, or —(CH2)n-aryl is optionally substituted with one or more —OH, halogen, —NO2, oxo, —CN, —R5, —OR5, —NR5R6, —SR5, —S(O)2NR5R6, —S(O)2R5, —NR5S(O)2NR5R6, —NR5S(O)2R6, —S(O)NR5R6, —S(O)R5, —NR5S(O)NR5R6, —NR5S(O)R6, —C(O)NR5R6, —NR5C(O)R6, heterocycle, aryl, heteroaryl, —(CH2)nOH, —C1-C6alkyl, —CF3, —CHF2, or —CH2F;
R2 is independently —H, —NH2, —ORb, —CN, —C1-C6alkyl, —C2-C6alkenyl, —C4-C8cycloalkenyl, —C2-C6alkynyl, halogen, —C(O)ORb, —C3-C8cycloalkyl, or heterocyclyl containing 1-5 heteroatoms selected from the group consisting of N, S, P, and O; wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, cycloalkyl, or heterocyclyl is optionally substituted with one or more —OH, halogen, —NO2, oxo, —R5, —OR5, —NR5R6, —SR5, —S(O)2NR5R6, —S(O)2R5, —NR5S(O)2NR5R6, —NR5S(O)2R6, —S(O)NR5R6, —S(O)R5, —NR5S(O)NR5R6, —NR5S(O)R6, heterocycle, aryl, or heteroaryl;
R3 combines with Ra to form a 3- to 12-membered monocyclic or polycyclic heterocycle or a 5- to 12-membered spiroheterocycle, wherein each heterocycle or spiroheterocycle is optionally substituted with one or more —C1-C6alkyl, halogen, —OH, —ORb, —NH2, —NHRb, optionally substituted heteroaryl, optionally substituted heterocyclyl, —(CH2)nNH2, —(CH2)nOH, —COORb, —CONHRb, —CONH(CH2)nCOORb, —NHCOORb, —CF3, —CHF2, —CH2F, or ═O; wherein the heteroaryl and heterocyclyl are optionally substituted with —CN;
R5 and R6 are independently, at each occurrence, —H, —C1-C6alkyl, —C2-C6alkenyl, —C4-C8cycloalkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, a monocyclic or polycyclic 3- to 12-membered heterocycle, —OR7, —SR7, halogen, —NR7R8, —NO2, —CF3, or —CN;
R7 and R8 are independently, at each occurrence, —H, —C1-C6alkyl, —C2-C6alkenyl, —C4-C8cycloalkenyl, —C2-C6alkynyl, —C3-C8cycloalkyl, —ORb, or a monocyclic or polycyclic 3- to 12-membered heterocycle, wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, cycloalkyl, or heterocycle is optionally substituted with one or more —OH, —SH, —NH2, —NO2, or —CN;
m is independently 1, 2, 3, 4, 5 or 6; and
n is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10.
|