| CPC C07D 495/04 (2013.01) [A61P 35/00 (2018.01)] | 27 Claims |
|
1. A method of treating a disease or condition in a subject, comprising administering to the subject a therapeutically effective amount of a compound of Formula (II-A):
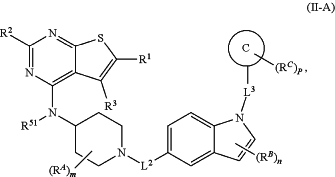 or a pharmaceutically acceptable salt thereof, wherein:
C is selected from C3-12 carbocycle and 3- to 12-membered heterocycle;
L2 is selected from bond, —C(O)—, —C(O)O—, —C(O)N(R51)—, —C(O)N(R51)C(O)—, —C(O)N(R51)C(O)N(R51)—, —C(NR51)—, —S(O)2—, —S(O)O—, —S(O)—, —S(O)2O—, —S(O)2N(R51)—, —S(O)N(R51)—, alkylene, alkenylene, alkynylene, heteroalkylene, heteroalkenylene, and heteroalkynylene, wherein each of the alkylene, alkenylene, alkynylene, heteroalkylene, heteroalkenylene, and heteroalkynylene is optionally substituted with one or more R50;
L3 is selected from alkylene, alkenylene, and alkynylene, each of which is substituted with one or more R56 and optionally further substituted with one or more R50;
R1 and R3 are each independently selected from hydrogen and R50;
R2 is R50;
RA, RB, and RC are each independently selected at each occurrence from R50, or two RA groups, two RB groups, or two RC groups attached to the same atom or different atoms can together optionally form a bridge or ring;
m, n, and p are each independently an integer from 0 to 6;
R50 is independently selected at each occurrence from:
halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(—O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, and —P(O)(R52)2, or two R50 groups attached to the same carbon are taken together to form ═O, =S, or =N(R52);
C1-10 alkyl, C2-10 alkenyl, and C2-10 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents selected from halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C3-12 carbocycle, and 3- to 12-membered heterocycle; and
C3-12 carbocycle and 3- to 12-membered heterocycle;
wherein each C3-12 carbocycle and 3- to 12-membered heterocycle in R50 is optionally substituted with one or more substituents independently selected from halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
R51 is independently selected at each occurrence from:
hydrogen, —C(O)R52, —C(O)OR52, —C(O)N(R52)2, and —C(O)NR53R54; and
C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl, each of which is optionally substituted at each occurrence with one or more substituents independently selected from halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C3-12 carbocycle, and 3- to 12-membered heterocycle; and
C3-12 carbocycle and 3- to 12-membered heterocycle;
wherein each C3-12 carbocycle and 3- to 12-membered heterocycle in R51 is optionally substituted with one or more substituents independently selected from halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl;
R52 is independently selected at each occurrence from hydrogen; and C1-20 alkyl, C2-20 alkenyl, C2-20 alkynyl, C1-6 heteroalkyl, C3-12 carbocycle, and 3- to 12-membered heterocycle, each of which is optionally substituted by halogen, —CN, —NO2, —NH2, —NHCH3, —NHCH2CH3, ═O, —OH, —OCH3, —OCH2CH3, C3-12 carbocycle, or 3- to 6-membered heterocycle;
R53 and R54 are taken together with the nitrogen atom to which they are attached to form a heterocycle;
R56 is independently selected at each occurrence from:
—NO2, —OR59, —SR52, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, C1-10 alkyl, C2-10 alkenyl, C2-10 alkynyl, C3-12 carbocycle, and 3- to 12-membered heterocycle, or two R56 groups attached to the same carbon are taken together to form ═O, ═S, or ═N(R52);
wherein each C1-10 alkyl, C2-10 alkenyl, and C2-10 alkynyl in R56 is optionally substituted at each occurrence with one or more substituents independently selected from halogen, —NO2, —CN, —OR59, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C3-12 carbocycle, and 3- to 12-membered heterocycle;
wherein each C3-12 carbocycle and 3- to 12-membered heterocycle in R56 is optionally substituted with one or more substituents independently selected from halogen, —NO2, —CN, —OR52, —SR52, —N(R52)2, —NR53R54, —S(═O)R52, —S(═O)2R52, —S(═O)2N(R52)2, —S(═O)2NR53R54, —NR52S(═O)2R52, —NR52S(═O)2N(R52)2, —NR52S(═O)2NR53R54, —C(O)R52, —C(O)OR52, —OC(O)R52, —OC(O)OR52, —OC(O)N(R52)2, —OC(O)NR53R54, —NR52C(O)R52, —NR52C(O)OR52, —NR52C(O)N(R52)2, —NR52C(O)NR53R54, —C(O)N(R52)2, —C(O)NR53R54, —P(O)(OR52)2, —P(O)(R52)2, ═O, ═S, ═N(R52), C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, and C2-6 alkynyl; and
further wherein R56 optionally forms a bond to ring C; and
R59 is independently selected at each occurrence from C1-20 alkyl, C2-20 alkenyl, C2-20 alkynyl, C1-6 heteroalkyl, C3-12 carbocycle, and 3- to 12-membered heterocycle, each of which is optionally substituted by halogen, —CN, —NO2, —NH2, —NHCH3, —NHCH2CH3, ═O, —OH, —OCH3, —OCH2CH3, C3-12 carbocycle, or 3- to 6-membered heterocycle,
wherein when R56 is —CH3, L3 is not further substituted with —OH, —NH2, or —CN; and
wherein the disease or condition comprises leukemia, hematologic malignancy, myelodysplastic syndrome, myelodysplastic/myeloproliferative neoplasm, solid tumor cancer, prostate cancer, breast cancer, liver cancer, brain tumor, or diabetes.
|