| CPC A61K 31/664 (2013.01) [A61K 38/29 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07F 9/3886 (2013.01)] | 5 Claims |
|
1. A compound, comprising:
A-Y—B,
wherein:
A is
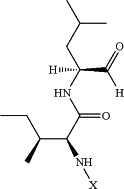 and X is selected from the group consisting of: H and
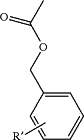 Y is a linker of formula NR1;
R′ is H, CH3, alkyl, halogen, CF3, CN, OH, OCH3, or O-alkyl;
wherein R1 is NR2R3, NR2S(═O)2R3 or R2OR3;
R2 and R3 are independently selected from H, C1-C10 alkyl, C1-C10 alkoxy, C6H5OR4, and wherein R2 and R3 are not both H,
R4 is C1-C10 alkyl, C1-C10 alkoxy, carbonyl, or amide; and
B is at least one bisphosphonate optionally substituted with OH, halogen, CH3, NH2,
or a pharmaceutically acceptable salt thereof.
|