| CPC A61B 17/3439 (2013.01) [A61B 17/3468 (2013.01); A61B 17/3498 (2013.01); A61F 2/2436 (2013.01); A61L 29/14 (2013.01); A61M 25/0662 (2013.01); A61M 29/00 (2013.01); A61M 2025/0024 (2013.01); A61M 2039/0626 (2013.01)] | 20 Claims |
|
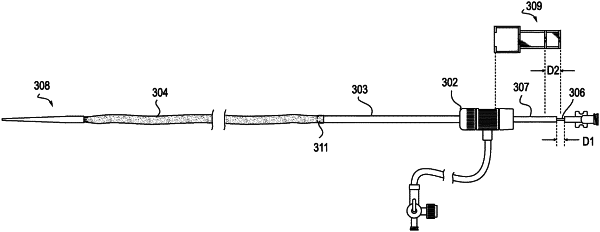
12. A method comprising:
deploying an introducer sheath in a blood vessel;
passing a device through a proximal rigid section of the introducer sheath into a distal flex section of the introducer sheath;
passing the device through the distal flex section to a delivery location within the blood vessel, wherein the device causes the distal flex section to locally expand solely at a location where the device is positioned within the distal flex section; and
preventing the distal flex section from inverting during the passing the device through the distal flex section.
|