| CPC C12Q 1/70 (2013.01) [B01L 7/52 (2013.01); C12M 47/06 (2013.01); B01L 2300/1805 (2013.01); B01L 2300/1883 (2013.01)] | 20 Claims |
|
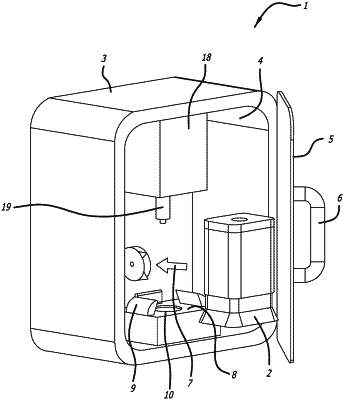
1. An infectious disease screening device for rapid detection of an infectious agent present in a sample fluid, the screening device comprising:
a substrate which is at least partly composed of silicon, the substrate comprising a sonication chamber having a sample inlet and a sample outlet;
an ultrasonic transducer in ultrasonic communication with the sonication chamber, wherein the ultrasonic transducer generates ultrasonic waves in a frequency range of approximately 2800 kHz to approximately 3200 kHz to lyse cells in the sample fluid within the sonication chamber;
a controller comprising:
an AC driver which generates an AC drive signal at a frequency within the frequency range of approximately 2800 kHz to approximately 3200 kHz and outputs the AC drive signal to drive the ultrasonic transducer,
an active power monitor which monitors active power used by the ultrasonic transducer when the ultrasonic transducer is driven by the AC drive signal, wherein the active power monitor provides a monitoring signal which is indicative of the active power used by the ultrasonic transducer;
a processor which controls the AC driver and receives the monitoring signal from the active power monitor; and
a memory storing instructions which, when executed by the processor, cause the processor to:
A. control the AC driver to output the AC drive signal to the ultrasonic transducer at a frequency within the frequency range;
B. calculate the active power being used by the ultrasonic transducer based on the monitoring signal;
C. control the AC driver to modulate the AC drive signal to maximize the active power being used by the ultrasonic transducer;
D. store a record in the memory of the maximum active power used by the ultrasonic transducer and the frequency of the AC drive signal;
E. repeat steps A-D for a predetermined number of iterations with the ultrasonic transducer being driven at a plurality of different frequencies across the frequency range of approximately 2800 kHz to approximately 3200 kHz;
F. identify from the records stored in the memory an optimum frequency for the AC drive signal which is the frequency of the AC drive signal at which the maximum active power is used by the ultrasonic transducer; and
G. control the AC driver to output the AC drive signal to the ultrasonic transducer at the optimum frequency,
wherein the substrate further comprises:
a reagent chamber having an inlet and an outlet, the inlet being coupled with the sample outlet of the sonication chamber to permit at least part of the sample fluid to flow from the sonication chamber to the reagent chamber so that the sample fluid mixes with a liquid PCR reagent in the reagent chamber,
wherein the substrate further comprises:
an extreme rRT-PCR cycler comprising:
a channel defining a fluid flow path between a channel inlet and a channel outlet, the channel inlet being coupled with the outlet of the reagent chamber to receive at least part of the sample fluid flowing into the channel from the reagent chamber, wherein the channel is formed from a plurality of interconnected turns which provide a plurality of channel sections across the surface of the substrate, wherein each channel section of the plurality of channel sections comprises a first channel portion, a second channel portion and a third channel portion;
a first heating element proximate each first channel portion;
a second heating element proximate each second channel portion; and
a third heating element proximate each third channel portion, wherein the controller controls:
the first heating element to heat the sample fluid flowing along each first channel portion,
the second heating element to heat the sample fluid flowing along each second channel portion, and
the third heating element to heat the sample fluid flowing along each third channel portion to perform extreme rRT-PCR on the sample fluid;
wherein the device further comprises:
an infectious agent detection apparatus which is in communication with and coupled to the channel outlet, wherein the infectious agent detection apparatus detects a presence of an infectious agent that causes the infectious disease in the sample fluid flowing out of the channel outlet, wherein the infectious agent detection apparatus provides an output which is indicative of whether or not the detection apparatus detects the presence of the infectious agent in the sample fluid.
|