| CPC C08G 18/242 (2013.01) [C08G 18/289 (2013.01); C08G 18/4825 (2013.01); C08G 18/778 (2013.01); C08G 2150/00 (2013.01); C08G 2170/00 (2013.01); C08G 2190/00 (2013.01)] | 13 Claims |
|
1. A process for producing alkoxysilane-containing polyurethanes
comprising the step of reacting
a compound containing at least one NCO group with a compound containing at least one Zerewitinoff-active H atom in the presence of a catalyst component,
wherein the compound containing at least one NCO group and/or the compound containing
at least one Zerewitinoff-active H atom contain at least one alkoxysilane group,
to afford an alkoxysilane-containing polyurethane,
wherein
the reaction is at least periodically performed at a temperature of >50 ° C. and in that the catalyst component comprises one or more cyclic tin compounds selected from the group of mono- or polycyclic tin compounds of the type:
1,1-di-“R”-5-“organyl”-5-aza-2,8-dioxa-l-stannacyclooctanes,
1,1-di-“R”-5-(N-“organyl”)aza-3,7-di-“organyl”-2,8-dioxa-1-stannacyclooctanes,
1,1-di-“R”-5-(N-“organyl”)aza-3,3,7,7-tetra-“organyl”-2,8-dioxa-1-stannacyclooctanes,
4,12-di-“organyl”-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecanes,
4,12-di-“organyl”-2,6,10,14-tetra-“organyl”-1,7,9,15-tetraoxa-4,12-diaza-8- stannaspiro[7.7]pentadecanes,
4,12-di-“organyl”-2,2,6,6,10,10,14,14-octa-“organyl”-1,7,9,15-tetraoxa-4,12-diaza-8- stannaspiro[7.7]pentadecanes,
wherein “R” represents D *, L3 or L4 and “organyl” represents R1 as defined below:
R1 represents a saturated or unsaturated, linear or branched, aliphatic or cycloaliphatic radical or an optionally substituted aromatic or araliphatic radical which has up to 20 carbon atoms and may optionally contain heteroatoms from the group of oxygen,
sulfur and nitrogen, or represents hydrogen or the radical
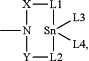 or R1 and L3 together represent —Z—L5—;
D* represents —O— or —S—;
X, Y and Z represent identical or different radicals selected from alkylene radicals of the formulae —C(R2)(R3)—, —C(R2)(R3)—C(R4)(R5)— or —C(R2)(R3)—C(R4)(R5)—C(R6)(R7)— or ortho-arylene radicals of the formulae
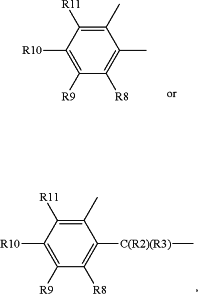 wherein R2 to R11 independently of one another represent saturated or unsaturated, linear or branched, aliphatic or cycloaliphatic or optionally substituted aromatic or araliphatic radicals which have up to 20 carbon atoms and may optionally contain heteroatoms from the group of oxygen, sulfur and nitrogen, or represent hydrogen;
L1, L2 and L5 independently of one another represent —O—, —S—, —OC (═O)—, —OC(═S)—, —SC(=O)—, —SC(═S)—, —OS(═O)2O—, —OS(═O)2— or —N(R12)—,
wherein R12 represents a saturated or unsaturated, linear or branched, aliphatic or cycloaliphatic radical or an optionally substituted aromatic or araliphatic radical which has up to 20 carbon atoms and may optionally contain heteroatoms from the group of oxygen, sulfur and nitrogen, or represents hydrogen;
L3 and L4 independently of one another represent —OH, —SH, —OR13, —Hal, —OC(═O)R14, —SR15,
—OC(═S)R16, —OS(═O)2OR17, —OS(═O)2R18 or —NR19R20, or L3 and L4 together represent -L1—X—D—Y—L2—(D represents —O—, —S— or —N(R1)—),
wherein R13 to R20 independently of one another represent saturated or unsaturated, linear or branched, aliphatic or cycloaliphatic or optionally substituted aromatic or araliphatic radicals which have up to 20 carbon atoms and may optionally contain heteroatoms from the group of oxygen, sulfur and nitrogen, or represent hydrogen,
wherein the cyclic tin compound is one or more of the following compounds:
4,12-di-n-butyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane,
4,12-di-n-butyl-2,6,10,14-tetramethyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane,
2,4,6,10,12,14-hexamethyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane,
4,12-di-n-octyl-2,6,10,14-tetramethyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane,
4,12-di-n-octyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane,
4,12-dimethyl-1,7,9,15-tetraoxa-4,12-diaza-8-stannaspiro[7.7]pentadecane or mixtures thereof.
|